A woman in her fifties with a post-operative infection, generalised rash and organ failure
A woman in her fifties developed symptoms of infection and multiorgan failure a few days after surgery for breast cancer. Her skin rash was a potential indication of a rare condition.
A woman in her fifties was urgently admitted to the surgical department of her local hospital due to fever, reduced general condition and dyspnoea five days after day surgery for right-sided breast cancer including breast-conserving surgery and sentinel lymph node biopsy.
The woman had an existing diagnosis of type 1 diabetes mellitus, hypertension, migraine, COPD and fibromyalgia. She smoked fewer than 10 cigarettes daily. Her regular medications consisted of insulin pump therapy, enalapril 20 mg perorally, and acetylsalicylic acid 75 mg perorally.
Upon admission, the woman was awake and alert. She had a body temperature of 38.6°C, respiratory rate of 20/min, oxygen saturation of 97 %, pulse of 95/min and blood pressure of 133/56 mmHg, as well as slight redness and discharge from the surgical wound on her breast.
Blood tests showed CRP 332 mg/L (reference range < 5), leukocytes 10.1 ∙ 109/L (3.5–8.8), including neutrophil granulocytes 9.6 ∙ 109/L (1.5–7.3), creatinine 101 μmol/L (45–90) and eGFR 53 ml/min/1.7m2 (≥ 60). Arterial blood gas was pH 7.41 (7.35–7.45), pCO2 4.2 kPa (4.64–6.40), pO2 8.6 kPa (11.1–14.4) and lactate 1.1 mmol/L (0.7–1.6).
Post-operative wound infection is the most common complication after breast surgery. It can lead to a poorer cosmetic outcome, delay the initiation of adjuvant treatment and represent a burden for the patient. According to Norway's National Quality Register for Breast Cancer, the proportion of wound infections requiring antibiotic therapy in 2022 was 4.9 % (1).
The woman did not meet any of the quick Sequential Organ Failure Assessment Score (qSOFA) criteria, but she met three of the four criteria for systemic inflammatory response syndrome (SIRS) and showed signs of organ dysfunction, with dyspnoea and reduced renal function. Incipient sepsis was therefore suspected. Blood cultures, a urine sample and nasopharyngeal PCR were taken, and sepsis treatment was initiated with intravenous cloxacillin 2 g × 4 and gentamicin 420 mg × 1.
According to the Norwegian Directorate of Health's guidelines for antibiotic therapy in hospitals, intravenous cloxacillin 2 g × 6 and intravenous gentamicin 6 mg/kg × 1 are recommended for sepsis with suspected origin in the skin or soft tissue. Our patient was treated in line with this.
As there was no breast and endocrine surgeon at the hospital, the patient was transferred the same day to the university hospital where she had undergone the procedure. Here it was found that she had developed a pruritic, diffuse, symmetrical erythema on the thorax and extremities. An allergic drug reaction to antibiotics was suspected, and a switch was made to intravenous clindamycin 600 mg × 4 monotherapy, and desloratadine antihistamines 5 mg × 2 perorally were initiated.
Penicillin allergy is frequently reported but the actual incidence is < 1 % (2). Type 1 reactions occur within minutes and result in a severe anaphylactic reaction. Type 4 reactions are delayed hypersensitivity reactions, mediated via T cells in the skin, and usually present as a pruritic maculopapular rash within 1–2 weeks, which can be treated with antihistamines or steroids (2).
The day after the transfer, the patient's condition worsened. NEWS (National Early Warning Score), a scoring system for assessing vital functions, increased from 2 to 9. She was awake but not fully alert. Despite infusion of a total of 1 L Ringer's acetate and 2 L NaCl 9 mg/ml, as well as 250 ml NaCl of intravenous fluid, her blood pressure was 88/54 mmHg. Pulse was 99/min, respiratory rate 26/min, oxygen saturation 95 % in room air and body temperature 38.4°C. She was anuric, and blood tests showed acute renal failure with creatinine 258 μmol/L (45 - 90), eGFR 17 ml/min/1.7m2 (≥ 60) and carbamide 15 mmol/L (2.6–6.4). Electrolyte tests showed moderate hyponatraemia 128 mmol/L (137–145) and normal potassium 4.2 mmol/L (3.6–5.0). Arterial blood gas showed compensated metabolic acidosis with pCO2 3.9 kPa (4.64–6.40), lactate 4 mmol/L (0.7–1.6) and base excess - 4 (-3–3). Elevated CRP and leukocyte count were unchanged. She reported no muscle pain and creatine kinase was normal.
The findings raised suspicion of septic shock as she exhibited signs of circulatory failure, with low blood pressure and high lactate despite initial fluid resuscitation.
The woman was transferred to the ICU for vasopressor therapy. Here her respiration was stable and she was breathing spontaneously without the need for oxygen. She maintained mean arterial pressure > 65 mmHg with the highest dose of intravenous noradrenaline of 0.08 µg/kg/min. She received a Ringer's acetate infusion and had a sparse but increasing hourly urine output of 30 ml/h. Glucocorticoids were unnecessary as she responded rapidly and stabilised on vasopressors and fluids. A serious streptococcal or staphylococcal infection was suspected, and the antibiotic therapy was supplemented with infusion of cefotaxime 2 g × 3 (in addition to the already initiated clindamycin).
The patient's renal failure was interpreted as prerenal due to hypovolaemia and sepsis. Aminoglycosides like gentamicin, which the patient received a single dose of upon admission, are nephrotoxic and may have contributed to the deterioration of her renal function.
The woman also had diarrhoea, with 3–4 loose bowel movements per day. She was tested for COVID-19 and influenza, as well as Clostridium difficile, but all tests were negative. Liver function tests showed mild abnormalities, with ALAT 70 U/L (10–45), ASAT 60 U/L (15–35), GT 195 U/L (10–75) and ALP 330 U/L (35–105). Total bilirubin was normal at 17 µmol/L (< 26). No petechiae or ecchymoses were observed on the skin, and coagulation tests showed an INR of 1.2 (0.8–1.2), with platelets within the normal reference range. D-dimer was elevated, at 4.7 mg/L (< 0.5), aPTT was 62 seconds (22–30) and fibrinogen 6.4 g/L (1.7–4.2).
The patient was assessed for disseminated intravascular coagulation (DIC). Elevated D-dimer and aPTT strengthened the suspicion, while elevated fibrinogen, normal INR and platelets, as well as the absence of clinical signs of bleeding, reduced the suspicion. The patient therefore had a low ISTH (International Society on Thrombosis and Haemostasis) DIC score (2 points), and DIC was thus considered less likely.
The patient experienced increasing shortness of breath after transferral to the ICU. Chest x-ray revealed congestion and small amounts of bilateral pleural effusion. She also developed pitting oedema in all four extremities, and blood tests showed a proBNP level of 13,505 ng/L (≤ 287). Results of blood cultures taken on the first, second and fourth days of hospitalisation were available after five days and were negative. ECG showed supraventricular tachycardia with a normal axis, with no signs of ischemia or bundle branch block. Echocardiography showed good biventricular function with an ejection fraction of 58 %. No vegetations on the valves or other evidence of endocarditis were detected. Left atrium was enlarged, 51 ml/m2, right atrium was not enlarged, 30 ml/m2, and the patient had mild tricuspid regurgitation. Tricuspid gradient was measured at 2.7 m/s (< 2.8). It was concluded that the patient had diastolic dysfunction grade 2. She received diuretic treatment with furosemide 20 mg × 2 perorally, which showed good clinical efficacy.
On the day of admission, our patient developed multiorgan failure with anuric renal failure, liver impairment, diastolic heart failure and pulmonary congestion, as well as gastrointestinal symptoms. The patient's medical history and symptoms could potentially indicate bacteraemia with Staphylococcus aureus and endocarditis. However, transthoracic echocardiography showed no signs of endocarditis, and the patient had no heart murmur, no signs of thromboembolism and no predisposing factors. Blood cultures also showed no bacterial growth. Endocarditis was therefore quickly considered less likely, and a transoesophageal echocardiography was considered unnecessary.
The day after transferral, we also observed that the rash had spread to the whole body, and the skin on the palms of the hands and soles of the feet were starting to peel (Figure 1). The patient's medical history and clinical presentation led us to consider toxic shock syndrome. Despite only mild redness around the surgical wound on the breast, the breast surgeon decided to open and revise the wound under local anaesthesia in the ICU. There was no visible pus or fluid accumulation in the wound, and the tissue appeared healthy. Samples were taken from the wound for cultures, and the skin was only partially closed to allow any potential fluid to drain out.

After two days in the ICU, the patient's respiration and circulation were stable, and she was transferred to the ward. Due to a persistent body temperature above 38°C, symptoms of organ failure such as dyspnoea, renal failure and heart failure, as well as persistently elevated CRP (200 mg/L) and leukocytes (21 ∙ 109/L) for more than four days, re-surgery under general anaesthesia was decided on. A quadrantectomy was performed of the entire lower medial quadrant of the right breast, corresponding to the affected area, and more samples were taken for cultures.
After re-surgery, the patient's general condition improved significantly, the rash faded and the skin on the palms of the hands and feet peeled off. Renal function returned to normal after ten days. Infection markers gradually decreased: on the ninth day, CRP was 69 mg/L and leukocytes were 17.5 ∙ 109/L. Cultures from the surgical wound were available on the fifth day and showed a pure culture of Staphylococcus aureus. Antibiotics were changed to peroral dicloxacillin 1 g × 4, based on susceptibility testing results. The patient was discharged after 15 days of dicloxacillin (total duration of antibiotic therapy of 22 days) and tapering doses of furosemide.
At the outpatient check-up three weeks later, proBNP was 740 ng/L (≤ 287), and infection parameters, renal function and liver function were normal. The wound on the breast appeared innocuous, and secondary closing was performed under local anaesthesia in the outpatient clinic.
It was the scarlatiniform rash on the palms that caught our attention and led us to consider toxic shock syndrome. It is a very rare but life-threatening condition caused by superantigen-producing bacteria, typically Staphylococcus aureus or Streptococcus pyogenes (3).
Discussion
The incidence of staphylococcal and streptococcal toxic shock syndrome is approximately 0.5 and 0.4 per 100,000 per year, respectively (4). The aetiology with staphylococci was under the spotlight around the 1980s when the condition was frequently associated with the use of hyper-absorbent tampons. Today, the condition is generally not related to menstruation, but it can occur post-operatively, post-partum, or with IUDs, burns or skin and soft tissue infections. Mortality in menstruation-related cases is < 5 %, while in non-menstruation-related cases it can be as high as 22 % (4, 5).
The condition is caused by exotoxins that act as bacterial superantigens. Twenty-four staphylococcal and 12 streptococcal superantigens have been identified. Among the most common is toxic shock syndrome toxin-1 (TSST-1), which up to 50 % of Staphylococcus aureus strains can produce (6). Over 90 % of individuals develop antibodies against TSST-1 in adulthood (3).
In a normal immune response, antigens are eliminated by antigen-presenting cells and presented in major histocompatibility complex II (MHC class II) molecules to antigen-specific T cells. Only 0.01–0.001 % of T cells are specific to the relevant antigen and will be activated. However, superantigens are able to bind directly to MHC II molecules and then to a part of the T cell receptor found on all T cells. The exotoxins can thus activate up to 30 % of the T cells (Figure 2) (7).
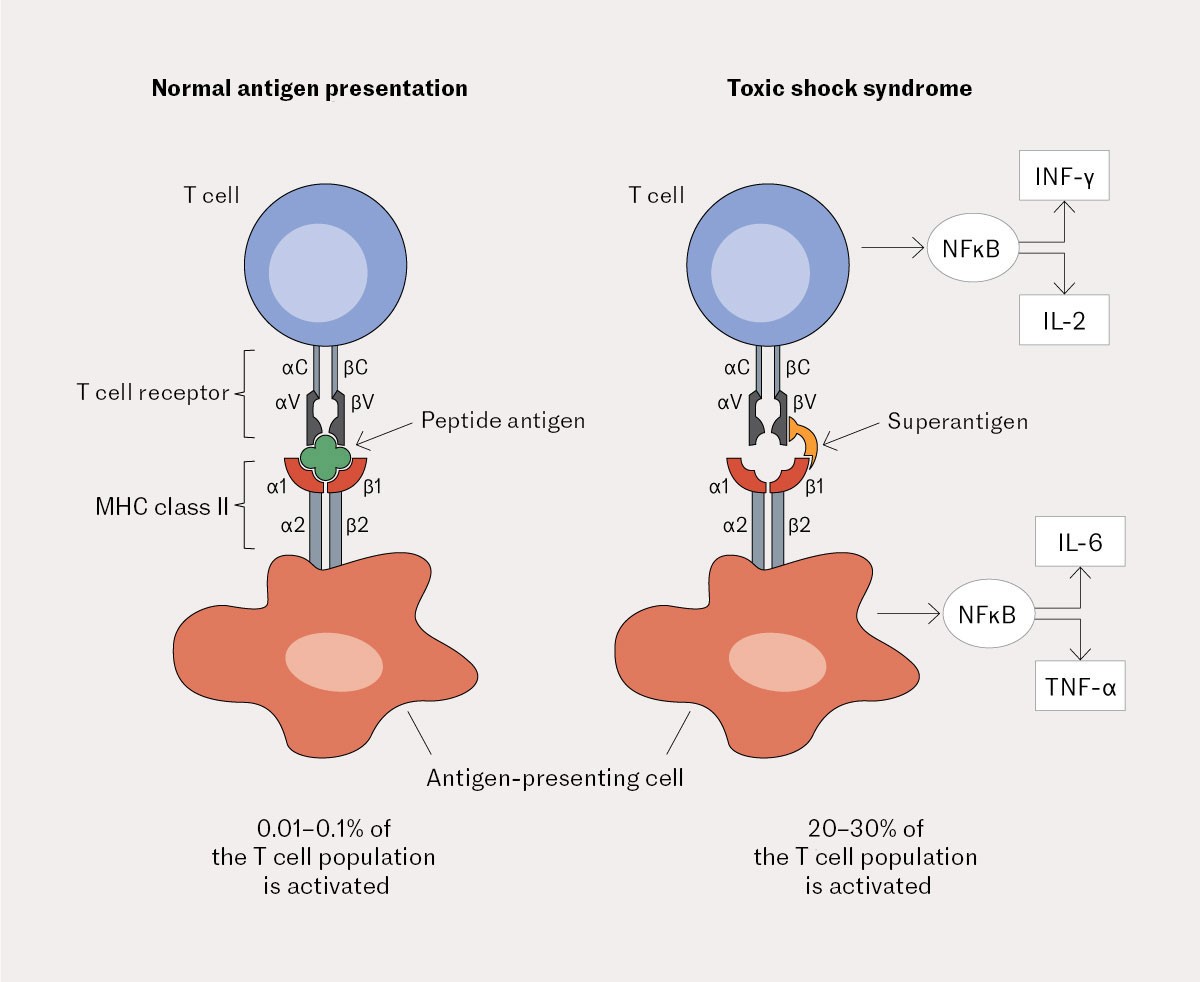
The activation leads to a massive release of proinflammatory cytokines such as lymphotoxin-alpha, interleukin-1, -2, and -6, interferon-gamma and tumour necrosis factor. Interleukin-1 can induce muscle proteolysis. The TSST-1 superantigen has a direct effect on blood vessels, resulting in capillary leakage and hypotension. Classic focal signs of infection can be modest, as superantigens inhibit macrophage infiltration in the infected area through overproduction of tumour necrosis factor (4, 8, 9) – as observed in our patient, for whom the findings at and around the surgical wound were sparse.
Symptoms of toxic shock syndrome develop rapidly (within 48 hours). The direct effects of toxins and cytokines, along with hypotension, result in multiorgan failure typically occurring 8–12 hours after symptom onset (10). Rapid diagnosis and treatment are therefore crucial to reduce mortality and morbidity. The diagnosis is based on clinical investigations, and there is no paraclinical test to differentiate toxic shock syndrome from other staphylococcal or streptococcal infections. The Centers for Disease Control and Prevention in the United States has developed diagnostic criteria, but these cannot be used to rule out toxic shock syndrome in individual suspected cases (11).
The classical presentation of diffuse erythema originates in the thorax and spreads to the extremities, particularly the palms of the hands and soles of the feet. This is typically followed by desquamation in these areas (Figure 1). Other typical symptoms and signs include high fever, low blood pressure, muscle pain (rhabdomyolysis), renal failure, liver failure, cardiomyopathy, pulmonary oedema and pleural effusion, vomiting and diarrhoea, headache, conjunctival, oropharyngeal and vaginal hyperaemia, anaemia, thrombocytopenia and disseminated intravascular coagulation (10). Important differential diagnoses are septic shock caused by other pathogens, adverse drug reaction, meningococci and inflammatory multisystem syndrome associated with COVID-19.
Treatment consists of supportive care to reverse the shock state, as well as surgery and antibiotics. Wounds may appear innocuous due to a weakened inflammatory response, but surgical debridement is still necessary (12). It is also important to remove any foreign bodies at suspected sites, such as tampons, IUDs, soiled dressings and piercings (9, 10, 13). According to the guidelines on antibiotic therapy, empiric antibiotic therapy is benzylpenicillin 2.4 g × 6 intravenously for streptococci, and cloxacillin 2 g × 6 intravenously for staphylococci (14). Using penicillin as a bactericidal agent inhibits the formation of bacterial cell walls, and it is important to add clindamycin 900 mg × 3 intravenously, which inhibits bacterial protein synthesis and thus the formation of toxins (6). This combination of beta-lactam antibiotics and clindamycin has been shown to have the best efficacy and should be the first-line treatment (6). Intravenous immunoglobulin can be given as adjunctive therapy, as it has an anti-inflammatory and immunomodulating effect, including inactivation of circulating superantigens (4, 13). Several observational studies point to lower mortality with the use of intravenous immunoglobulin, but the evidence is uncertain due to the absence of randomised controlled trials (4).
Over 90 % of individuals develop antibodies against TSST-1 by the age of 25. Toxic shock syndrome with TSST-1 only affects the 10 % who do not have antibodies (15). Half of these patients do not seroconvert and they have a reduced ability to produce antibodies after an illness, putting them at increased risk of a new episode (15).
Toxic shock syndrome is believed to be underdiagnosed as some patients only meet the criteria retrospectively (8). Today, the condition is not typically associated with menstruation, and toxic shock syndrome should be considered in recently operated patients who rapidly develop a high fever, rash and multiorgan failure.
The patient has consented to publication of this article.
The article has been peer-reviewed.
- 1.
Nasjonalt kvalitetsregister for brystkreft. Årsrapport 2022 med resultater og forbedringstiltak fra Nasjonalt kvalitetsregister for brystkreft. https://www.kvalitetsregistre.no/sites/default/files/2023-06/%C3%85rsrapport%202022%20Nasjonalt%20kvalitetsregister%20for%20brystkreft.pdf Accessed 22.2.2024.
- 2.
Berild D. Penicillinallergi. https://antibiotikaiallmennpraksis.no/index.php?action=topic&item=pJHa548e Accessed 22.2.2024.
- 3.
Gottlieb M, Long B, Koyfman A. The evaluation and management of toxic shock syndrome in the emergency department: a review of the literature. J Emerg Med 2018; 54: 807–14. [PubMed][CrossRef]
- 4.
Burnham JP, Kollef MH. Understanding toxic shock syndrome. Intensive Care Med 2015; 41: 1707–10. [PubMed][CrossRef]
- 5.
Descloux E, Perpoint T, Ferry T et al. One in five mortality in non-menstrual toxic shock syndrome versus no mortality in menstrual cases in a balanced French series of 55 cases. Eur J Clin Microbiol Infect Dis 2008; 27: 37–43. [PubMed][CrossRef]
- 6.
Annane D, Clair B, Salomon J. Managing toxic shock syndrome with antibiotics. Expert Opin Pharmacother 2004; 5: 1701–10. [PubMed][CrossRef]
- 7.
Celie KB, Colen DL, Kovach SJ. Toxic Shock Syndrome after Surgery: Case Presentation and Systematic Review of the Literature. Plast Reconstr Surg Glob Open 2020; 8. doi: 10.1097/GOX.0000000000002499. [PubMed][CrossRef]
- 8.
Hansen NS, Leth S, Nielsen LT. Toxic shock syndrome. Ugeskr Laeger 2020; 182.. [PubMed]
- 9.
Lappin E, Ferguson AJ. Gram-positive toxic shock syndromes. Lancet Infect Dis 2009; 9: 281–90. [PubMed][CrossRef]
- 10.
Murray RJ. Recognition and management of Staphylococcus aureus toxin-mediated disease. Intern Med J 2005; 35 (Suppl 2): S106–19. [PubMed][CrossRef]
- 11.
Centers for Disease Control and Prevention. Case definitions for infectious conditions under public health surveillance. MMWR Recomm Rep 1997; 46 (RR-10): 1–55. [PubMed]
- 12.
Spaulding AR, Salgado-Pabón W, Kohler PL et al. Staphylococcal and streptococcal superantigen exotoxins. Clin Microbiol Rev 2013; 26: 422–47. [PubMed][CrossRef]
- 13.
Wilkins AL, Steer AC, Smeesters PR et al. Toxic shock syndrome - the seven Rs of management and treatment. J Infect 2017; 74 (Suppl 1): S147–52. [PubMed][CrossRef]
- 14.
Helsedirektoratet. Antibiotika i sykehus. https://www.helsedirektoratet.no/retningslinjer/antibiotika-i-sykehus/hud-og-blotdelsinfeksjoner Accessed 1.2.2024.
- 15.
Parsonnet J, Hansmann MA, Delaney ML et al. Prevalence of toxic shock syndrome toxin 1-producing Staphylococcus aureus and the presence of antibodies to this superantigen in menstruating women. J Clin Microbiol 2005; 43: 4628–34. [PubMed][CrossRef]