The prevalence of renal anaemia increases with progression of chronic kidney disease. Roxadustat, a new oral alternative to conventional treatment with injectable erythropoiesis-stimulating agents, can cause central hypothyroidism.
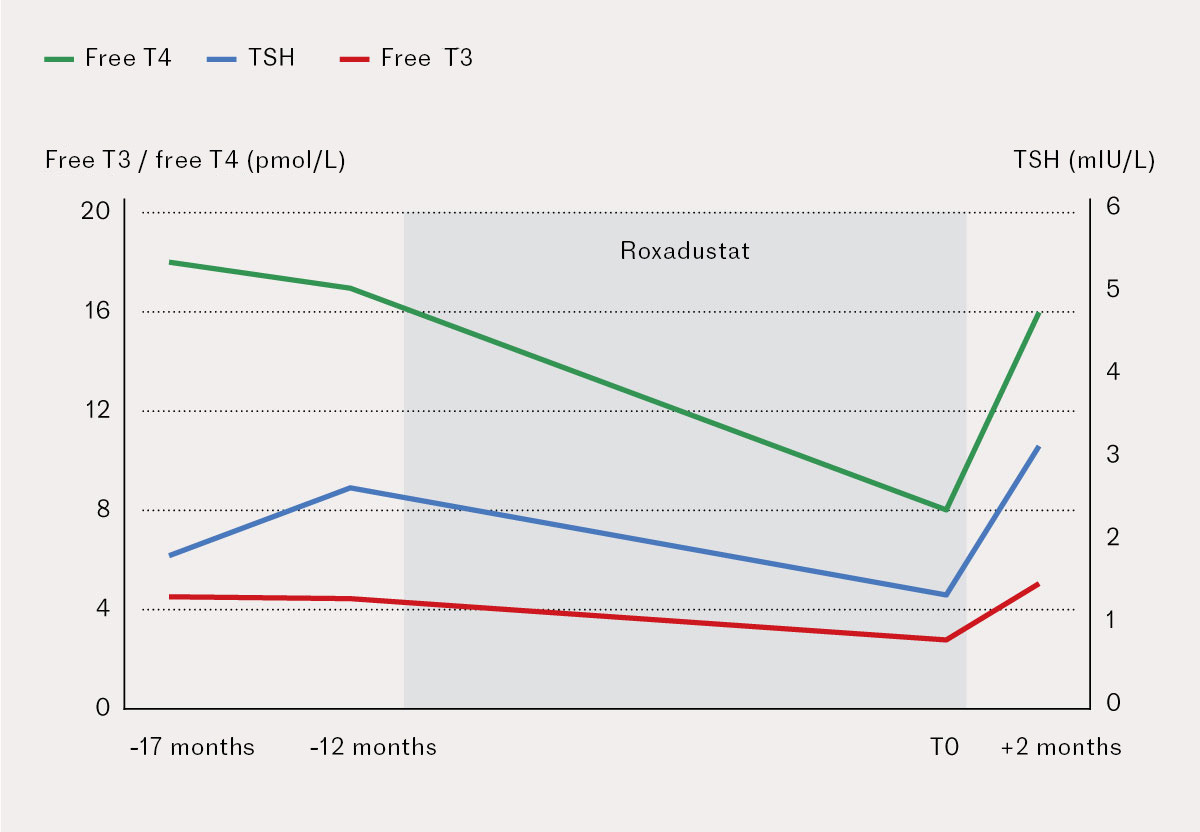
A woman in her sixties with pre-dialysis (stage 5) chronic kidney disease as a result of hypertension and type 2 diabetes had steadily decreasing haemoglobin (9.7 g/dL (reference range: 11.7–15.3)) at check-ups, despite normal iron stores and with no extrarenal cause of the anaemia. She received parenteral iron due to gastrointestinal adverse effects of oral supplementation, and was otherwise well compensated with no uraemic symptoms. She had no goitre or known thyroid disease, thyroid function tests were normal (thyroid-stimulating hormone (TSH): 2.6 mIU/L (0.27–4.2), free T4: 17 pmol/L (12–22), free T3: 4.4 pmol/L (3.1–6.8)), and she was not using any medications known to affect the thyroid (amiodarone, lithium, etc.).
Since the patient had persistent anaemia despite normal iron stores, initiation of roxadustat was found to be indicated at a dose of 70 mg three times weekly, with a target haemoglobin of 10–12 g/dL. Follow-up blood tests three months later revealed improved haemoglobin of 11.2 g/dL, at which point the roxadustat dose was provisionally reduced to 70 mg twice weekly for five months, before being increased again to 70 mg three times weekly.
The patient was in work, maintained a stable weight and did not report any symptoms spontaneously that would suggest hypothyroidism during treatment. Since it is known that roxadustat can cause hypothyroidism in rare cases, routine testing of thyroid parameters was performed after eleven months of treatment. The results of these tests were consistent with central hypothyroidism (TSH: 1.3 mIU/L, free T4: 8 pmol/L, free T3: 2.7 pmol/L) (Figure 1). Other pituitary axis results were normal (ACTH: 6.6 pmol/L (<10), cortisol: 310 nmol/L (70–690), FSH: 65 IU/L (26–135), LH: 61 IU/L (7.7–59), oestradiol: <0.09 nmol/L (< 0.51), prolactin: 343 mIU/L (102–496), IGF-1: 19 nmol/L (4.0–22)). On further questioning however, it turned out that the patient had been experiencing fairly typical symptoms (increased fatigue, reduced ability to concentrate, constipation and feeling cold). Roxadustat was discontinued due to the suspected adverse drug reaction while awaiting further investigation. Blood tests six weeks later found thyroid parameters had returned to normal (TSH: 3.1 mIU/L, free T4: 16 pmol/L, free T3: 5.0 pmol/L). The patient was then started on a conventional erythropoiesis-stimulating agent (methoxy polyethylene glycol-epoetin beta) to treat her renal anaemia. She is on the transplant waiting list, and receiving close monitoring every other month at the renal outpatient clinic with blood tests.
Discussion
Hypothyroidism is a very common chronic condition, with primary hypothyroidism (failure of the thyroid gland) accounting for more than 99 % of cases (1). By contrast, central hypothyroidism (caused by insufficient TSH stimulation of the thyroid gland) accounts for a small proportion of cases and is often accompanied by deficiency in other pituitary axes (hypopituitarism), such as cortisol, growth hormone and sex hormone deficiency. Hypopituitarism is a rare condition that is usually caused by pituitary adenoma or tumours/cysts in or near the pituitary gland, or as a result of surgery, radiotherapy, infiltrative disease or modern immunotherapy (2).
Isolated central hypothyroidism is extremely rare and generally congenital (3). It can be challenging to diagnose because TSH will usually be low or 'inappropriately' normal, and therefore the diagnosis is dependent on free T4 and free T3. A key differential diagnosis is non-thyroidal illness syndrome, a condition in which acute illness also leads to a decrease in free thyroid hormones along with low TSH.
Anaemia is a common complication in patients with chronic kidney disease, with a prevalence ranging from 15 % with any stage of CKD to over 50 % in patients with stage 5 (4). Renal anaemia is associated with increased mortality (5), and thus it is treated with iron supplementation and erythropoiesis-stimulating agents, with a target haemoglobin above 10.0 g/dL (6).
An oral alternative to erythropoiesis-stimulating agents has recently become available, roxadustat (7), which is a hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PH). In Norway, it was used in approximately 281 patients with renal anaemia in 2023 (8). Through the reversible inhibition of HIF-PH, the stimulating effects of roxadustat include increased plasma levels of endogenous erythropoietin, reduction in hepcidin, an increased number of transporter proteins and increased iron uptake. Overall, this results in improved iron bioavailability, increased haemoglobin and increased red cell mass (7).
Several case reports have been published with a similar clinical course to that of our patient (9, 10), and a recent retrospective study (11) found significantly lower thyroid parameters (TSH, free T4 and free T3) in patients treated with roxadustat compared with erythropoietin. The likely cause is roxadustat's structural similarity to triiodothyronine (T3), which results in reduced secretion of TSH via a feedback mechanism (the receptor THRβ) in the pituitary gland. This in turn leads to reduced stimulation of the thyroid gland and a net reduction in the release of thyroid hormones (T4 and T3) into the blood stream (12).
Patient's perspective
I found I was getting more tired and forgetful, and I had difficulty concentrating. I also developed dry skin and mucous membranes, decreased appetite, constipation, and muscle and joint pain. One month after stopping roxadustat, I noticed an improvement in my symptoms.
We consider it highly likely that our patient had transient isolated central hypothyroidism secondary to treatment with roxadustat. Hypothyroidism can cause significant symptoms, and therefore it is imperative to monitor the thyroid function of patients receiving roxadustat treatment. The analysis of free T3 and free T4 in addition to TSH is essential, since the condition may not necessarily be detected with TSH alone. The case has been notified as an adverse drug reaction to the Norwegian Medicines Agency.
The patient has consented to the publication of the article.
The article has been peer-reviewed.
- 1.
Chiovato L, Magri F, Carlé A. Hypothyroidism in Context: Where We've Been and Where We're Going. Adv Ther 2019; 36 (Suppl 2): 47–58. [PubMed][CrossRef]
- 2.
Fleseriu M, Christ-Crain M, Langlois F et al. Hypopituitarism. Lancet 2024; 403: 2632–48. [PubMed][CrossRef]
- 3.
Persani L. Clinical review: Central hypothyroidism: pathogenic, diagnostic, and therapeutic challenges. J Clin Endocrinol Metab 2012; 97: 3068–78. [PubMed][CrossRef]
- 4.
Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One 2014; 9. doi: 10.1371/journal.pone.0084943. [PubMed][CrossRef]
- 5.
Foley RN, Parfrey PS, Harnett JD et al. The impact of anemia on cardiomyopathy, morbidity, and and mortality in end-stage renal disease. Am J Kidney Dis 1996; 28: 53–61. [PubMed][CrossRef]
- 6.
Official journal of the international society of nephrology. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-Anemia-Guideline-English.pdf Accessed 29.10.2024.
- 7.
Chen N, Hao C, Peng X et al. Roxadustat for Anemia in Patients with Kidney Disease Not Receiving Dialysis. N Engl J Med 2019; 381: 1001–10. [PubMed][CrossRef]
- 8.
Folkehelseinstituttet. Legemiddelstatistikk per ATC-kode. https://statistikk.fhi.no/lmr/ Accessed 15.10.2024.
- 9.
Tokuyama A, Kadoya H, Obata A et al. Roxadustat and thyroid-stimulating hormone suppression. Clin Kidney J 2021; 14: 1472–4. [PubMed][CrossRef]
- 10.
Ichii M, Mori K, Miyaoka D et al. Suppression of thyrotropin secretion during roxadustat treatment for renal anemia in a patient undergoing hemodialysis. BMC Nephrol 2021; 22: 104. [PubMed][CrossRef]
- 11.
Cheng Y, Xiang Q, Cao T et al. Suppression of thyroid profile during roxadustat treatment in chronic kidney disease patients. Nephrol Dial Transplant 2023; 38: 1567–70. [PubMed][CrossRef]
- 12.
Yao B, Wei Y, Zhang S et al. Revealing a Mutant-Induced Receptor Allosteric Mechanism for the Thyroid Hormone Resistance. iScience 2019; 20: 489–96. [PubMed][CrossRef]