A man in his sixties with life-threatening febrile illness after travel abroad
A man in his sixties was in East Africa for work when he suddenly felt generally unwell, lethargic and feverish. He flew home to Norway earlier than planned and was admitted to hospital shortly after with a fever and multi-organ failure. Investigations revealed a life-threatening illness.
A Norwegian man in his sixties was in East Africa for work. He had previously been generally healthy and did not smoke. He used mosquito nets and took atovaquone-proguanil (Malarone) tablets 250 mg/100 mg × 1 daily for malaria prevention but had no other regular medications. He was vaccinated against yellow fever, typhoid fever, meningococcal disease (groups A, C, W-135 and Y), hepatitis A and B, diphtheria, tetanus, pertussis and polio. After nine weeks abroad, he became unwell. He felt feverish, lethargic and very ill. On day 2 of the illness he consulted a doctor, and blood smear microscopy found no malaria. The doctor prescribed doxycycline tablets 100 mg × 2 daily and advised the patient to drink plenty of water.
Falciparum malaria is common in Sub-Saharan Africa and can quickly become life-threatening. Mosquito nets and preventive treatment in tablet form reduce the risk but do not completely rule out malaria. Doxycycline was likely chosen to treat unspecified bacterial infection, and the drug is particularly effective against certain tropical febrile diseases, such as rickettsiosis and leptospirosis (1). Other important differential diagnoses include typhoid fever and mosquito-borne viral infections such as dengue fever.
The patient's condition quickly deteriorated despite treatment with doxycycline and ample fluid intake. He wanted to go home but was unable to book a flight for himself. He called his wife, who managed to book a flight for the same evening, on day 3 of the illness. His roommate had to accompany him to the airport, and the patient was so unwell that he was surprised he was allowed to board the plane. He remembered little of the actual journey. When he landed in Norway on day 4 of the illness, his wife drove him to his general practitioner, who sent him to Haukeland University Hospital.
Acute tropical infections should be managed in consultation with an infectious disease specialist, and patients in poor general condition should be hospitalised.
Upon admission, the patient was in poor general condition. He was oriented to time and place but somnolent and confused, and spoke incoherently at times in English, even though the staff were Norwegian. He had a fever of 39.8°C, tachypnoea with 26 breaths per minute, pulse of 114 beats per minute, blood pressure of 155/94 mmHg and oxygen saturation of 95 %. He exhibited yellowing of the sclera and had a slightly hyperaemic, blanching rash on his body (see video 1). He had reduced urine production and blood was visible in the urine. Otherwise, clinical findings were normal, including for examinations of the abdomen, heart and lung sounds. He did not have a stiff neck. Absence of eschar reduced the likelihood of rickettsiosis.
It was apparent that the patient had a severe infectious disease. Reduced urine production, jaundice and blood in the urine could suggest multi-organ failure, including renal failure and dysfunction of the liver and coagulation. The patient appeared septic, but due to his travel history, malaria and severe dengue fever were important differential diagnoses.
Blood tests upon admission showed haemoglobin 17.4 g/dL (normal range 13.4–17.0), low leukocyte count 3.3 × 109/L (4.1–9.8 × 109) and platelets 22 × 109/ L (145–348 × 109), CRP 216 mg/L (< 5), procalcitonin 4.7 µg/L (< 0.1), creatinine 108 µmol/L (60–105), bilirubin 96 µmol/L (< 20), alanine aminotransferase (ALT) 531 U/L (10–70), gamma-glutamyl transferase (GGT) 594 U/L (15–115), lactate dehydrogenase (LD) 860 U/L (105–205), alkaline phosphatase (ALP) 239 U/L (35–105), creatine kinase (CK) 2411 U/L (40–280), troponin T 64 ng/L (< 15), INR 1.3, D-dimer > 4 mg/ L (< 0.5) and normal fibrinogen 2.6 g/L (1.9–4.0). Blood samples were also taken for thin and thick smear microscopy and rapid tests for malaria and dengue fever.
The blood test results confirmed the clinical suspicion of renal, hepatic and coagulation system dysfunction as well as probable hepatitis. Troponin T levels were slightly elevated, but echocardiography showed no signs of myocarditis.
Severe Plasmodium falciparum infection could account for symptoms of fever, bloody urine, leukopenia, thrombocytopenia, jaundice and multi-organ failure. Malaria causes anaemia, but dehydration could explain the high haemoglobin levels. The combination of renal failure, jaundice and bleeding could be consistent with a diagnosis of leptospirosis. Lack of exposure to fresh water does not rule out this disease as it can also be contracted through contact with animals or sewage.
His symptoms could also be consistent with enteric fever caused by Salmonella enterica serotype typhi or paratyphi. The word typhos, which is Greek for smoke or fog, refers to the patient's confused state, and normotension is more indicative of enteric fever, where the amount of bacteria in the blood is relatively lower than with other gram-negative sepsis. Recent vaccination against S. typhi, absence of abdominal symptoms and paradoxical bradycardia made this diagnosis somewhat less likely. Severe dengue fever could fit with symptoms of haematuria, leukopenia and thrombocytopenia, and high haemoglobin levels could be due to fluid extravasation but tend to occur together with hypotension.
Severe viral haemorrhagic fevers such as Ebola, Marburg virus disease and yellow fever occur sporadically in East Africa, but importation is unlikely when there are no ongoing outbreaks. Additionally, the Stamaril vaccine also provides good protection against yellow fever. Rickettsioses are common causes of febrile illness in Africa (2), but spotted fever caused by Rickettsia africae rarely leads to serious illness, and the patient did not have eschar, which is common with both R. Africae and R. conorii.
The patient had been in the 'meningitis belt' (the area around the southern Sahara).
Meningococcal disease could account for mental impairment, coagulation and multi-organ failure. However, it was considered less likely due to the patient's meningococcal vaccination and the somewhat protracted clinical course without neck stiffness or petechiae.
From the time of admission, the patient received intravenous ampicillin 2 g × 4 and gentamicin 400 mg × 1 to treat sepsis and possible urinary tract infection in relation to haematuria. Ampicillin would also treat leptospirosis. The patient stopped taking doxycycline before flying home, and no specific treatment was given for rickettsial infection, which was less likely, and which should have improved somewhat following the doxycycline treatment abroad. The patient was admitted to the intermediate care unit in the Section for Infectious Diseases where he received supportive treatment with infusion of three litres of crystalloids, one litre of 5 % glucose and two units of platelet concentrate for the first 24 hours. He did not require vasopressor treatment or respiratory support.
The malaria rapid test was negative. Due to severe thrombocytopenia, the biomedical scientist performed microscopy with manual platelet counting. She was surprised to find something moving among the blood cells in the counting chamber (see video 2) and immediately called the infectious disease consultant on duty, who was still waiting for blood smears for microscopy.
We examined a drop of unstained blood on a microscope slide with a glass coverslip and found elongated parasites swimming in corkscrew movements (see videos 3 and 4). In a Giemsa-stained blood smear, we observed an abundance of elongated protozoa of the species Trypanosoma brucei lying freely among the blood cells.
There were approximately 5 parasites per 100 erythrocytes (Figure 1).
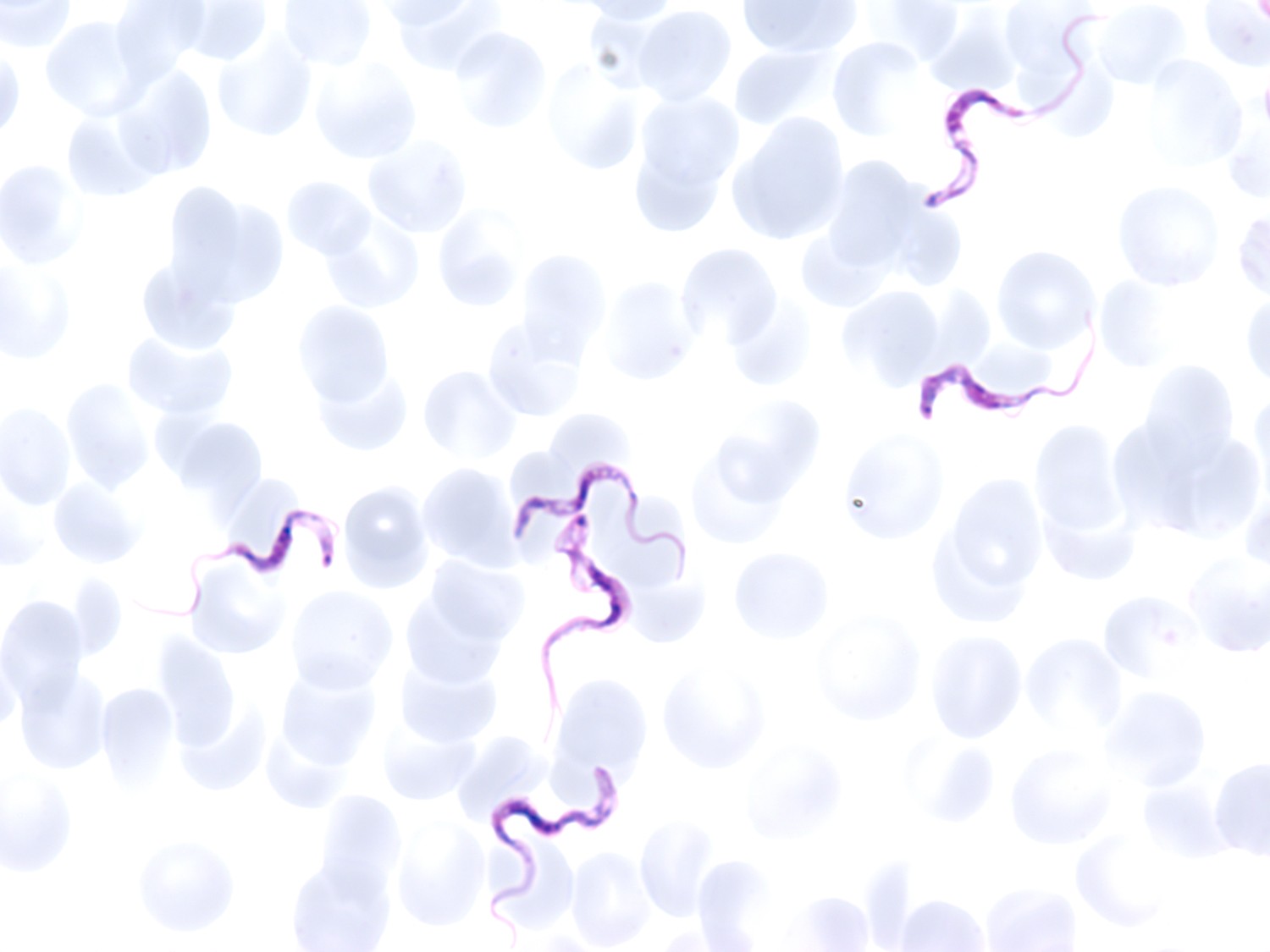
Trypanosoma brucei is the cause of African sleeping sickness, also known as human African trypanosomiasis. T. brucei are elongated protozoa, 13–33 µm long, that move with an undulating motion aided by a flagellum attached to the cell membrane (3). It is not possible to distinguish between the subspecies Trypanosoma brucei gambiense (found in Central and West Africa) and T.b. rhodesiense (found in East Africa) by microscopy.
African sleeping sickness is transmitted via a painful bite from tsetse flies, Glossina species (Figure 2), which look similar to clegs or horseflies. A chancre may develop at the site of the bite, where the parasites multiply in the following days. When the trypanosomes enter the bloodstream, the host develops a febrile illness (stage 1). Without treatment, the parasite eventually enters the brain, causing encephalitis (stage 2). The prognosis is very poor without treatment, although recent research has cast doubt on the notion that the disease is always fatal (4). Pharmacotherapy is determined based on the subspecies (T.b. rhodesiense or T.b. gambiense) and the clinical stage (whether encephalitis is present or not) (3).

The patient confirmed a painful bite from a tsetse fly during a safari trip in Murchison Falls, western Uganda, 11 days before he fell ill. He did not experience swelling or redness consistent with canker sores. Although the region is endemic for T. b. gambiense, the clinical picture of high fever, systemic involvement, multi-organ failure and high parasitaemia was more consistent with T. b. rhodesiense infection. Medications for East African sleeping sickness include suramin for the first stage and melarsoprol for the encephalitis stage (3). We did not have these in stock. On the day of admission (day 4 of illness), the patient was treated with a single dose of the antiprotozoal agent pentamidine (300 mg intravenously), which is effective for the first stage of T.b. gambiense infection but less effective for T.b. rhodesiense. The patient was critically ill, and antibiotics were successively changed to cefotaxime 2 g × 3 and then to meropenem 1 g × 3 intravenously, in view of potential co-infection with multi-drug resistant bacteria. We had suramin flown in from another university hospital, and on the day after admission (day 5 of illness) we initiated the correct treatment for East African sleeping sickness with intravenous suramin 1 g mixed in 500 mL of NaCl, with a planned five doses over three weeks. The quantity of parasites in blood smears decreased after just one dose of pentamidine, despite the patient experiencing increasing renal dysfunction (creatinine 189 μmol/L) and coagulation (12 platelets/nL) with increased INR 1.5 (< 1.1) and D-dimer > 4 mg/L (≤ 0.5) but normal fibrinogen 2.6 g/L (2.0–4.9).
T.b. rhodesiense encephalitis is the only infectious disease still treated with the highly toxic arsenic derivative melarsoprol. Previous complex, protracted dosing regimens have been replaced with a ten-day short-term regimen of melarsoprol infusions, 2.2 mg/kg daily. Around 10 % of patients develop arsenic-reactive encephalopathy, and about half of these die (5). Prophylactic prednisolone (1 mg/kg up to 40 mg daily) is used to reduce the risk of encephalopathy (6).
We ordered melarsoprol from the World Health Organization (WHO) in Geneva the day after the patient was admitted (day 5 of illness). Spinal fluid examination is used for staging, where the detection of parasites in the spinal fluid confirms stage 2 disease, and leukocyte count > 5 × 106/L indicates probable stage 2. We delayed lumbar puncture until day 8 of the illness, after the second dose of suramin, to reduce the parasite load in the blood and the risk of iatrogenic transmission of parasites into the spinal fluid.
No visible parasites were found in the spinal fluid, but leukocytes were 24 × 106/L (< 3), 99 % mononuclear cells, with normal levels of protein 0.4 g/L (0.15–0.5) and blood glucose 3 .3 mmol/L (2.2–4.4). Pleocytosis and slightly elevated immunoglobulin M in the spinal fluid (0.08 mg/dL, reference < 0.05) suggested stage 2 disease, but the absence of parasites in the spinal fluid suggested stage 1. We received the shipment of melarsoprol five days after the patient was admitted (day 9 of illness). We initiated prophylactic pre-treatment with prednisolone 40 mg daily while considering the potential toxicity of melarsoprol in light of the patient's remarkable clinical improvement with suramin, including regaining his mental clarity, as well as studies indicating the efficacy of stage 1 treatment with spinal fluid cell counts up to around 20 × 106/L.
After internal discussion and consultation with international experts, we decided to treat the patient for stage 1 disease with suramin alone, administered as 1 g intravenously five times over three weeks, corresponding to days 5, 7, 11, 18 and 26 of the illness. The Institute of Tropical Medicine in Antwerp subsequently confirmed that PCR analysis showed T.b. rhodesiense in the blood. Negative PCR in the spinal fluid supported the absence of encephalitis. We reported the case to the WHO, which had not previously recorded T.b. rhodesiense infection in this region.
After the second dose of suramin, the patient was fever-free on day 8 and gradually recovered, with normalisation of platelets on day 12, transaminases on day 18 and bilirubin on day 28. From day 7 of the illness, he had mild hypertension with blood pressure around 160/80 mmHg, and on day 8, proteinuria was detected (protein/creatinine ratio 67 mg/mmol (< 20), albumin/creatinine ratio 37 mg/mmol (< 3)). The patient received nifedipine extended-release tablets 20 mg daily from day 14 and was discharged on day 28 with a dose of 30 mg daily.
At the five-week follow-up, which included a lumbar puncture, he felt much better but still had a 'cotton wool sensation' in his head, and both the cell count (10 × 106/L) and IgM levels (0.12 mg/dL) in the spinal fluid were still slightly elevated. At the ten-week follow-up after the start of treatment, the patient felt completely healthy and his spinal fluid was normal, as was also the case at subsequent check-ups for the following two years. Seven years later, he still has a borderline high creatinine level (110 µmol/L) and is receiving nifedipine for hypertension but is otherwise clinically and mentally healthy.
Discussion
African sleeping sickness has caused major, deadly epidemics in the region between the Sahara and the Kalahari, where tsetse flies thrive, particularly in connection with forced population displacement and ecological changes during the colonial era (3). Around the turn of the millennium, there were around 300 000 annual cases of the disease. In the last two decades, screening, treatment (T.b. gambiense) and vector control (T.b. rhodesiense) have helped bring the disease under control, but it is feared that new epidemics may occur if control measures are disrupted due to war or mismanagement (3).
T.b. gambiense infection is primarily an anthroponosis in which the tsetse fly transmits the infection between humans. Infection with T.b. rhodesiense is mainly a zoonosis, where the parasite thrives in livestock such as cattle, goats and sheep, or wild animals such as antelopes (3). Two to three days after the bite, a painful, itchy canker can occur, which usually disappears within 1–2 weeks (3). The patient then enters a haemolymphatic first stage, with fever occurring at irregular intervals, enlarged glands, headache, myalgia, pruritus, lethargy and poor appetite, before progressing to the second stage with encephalitis (3). T.b. gambiense infection progresses over months and years from a febrile illness with enlarged cervical lymph nodes to chronic encephalitis characterised by neuropsychiatric symptoms and disturbed sleep patterns, and if left untreated, usually leads to coma and death, sometimes after up to three years of suffering (7).
A study from around 10 years ago showed that untreated T.b. gambiense infection is not always fatal as previously believed (4). The clinical manifestation of T.b. rhodesiense varies considerably, but it usually presents as a more acute, rapidly progressing, aggressive disease, with fever and pronounced systemic involvement, often with vital organ failure, myocarditis, hepatitis and pronounced neurological findings. Patients can die within weeks or months (8). Our patient had multi-organ failure on day 4 of his illness; 15 days after being bitten by a tsetse fly.
The disease is rare outside of Africa, but can occur among safari tourists (mainly T.b. rhodesiense) and migrants (T.b. gambiense). In the period 1990–2010, only 49 cases of T.b. rhodesiense – two of which were fatal – were reported in non-endemic areas, in addition to 19 non-fatal cases of T.b. gambiense infection (9).
African sleeping sickness is diagnosed by detecting trypanosomes on microscopy of lymph node aspirates (T.b. gambiense) or blood (usually for T.b. rhodesiense), while detection in spinal fluid confirms whether the patient has the encephalitic stage. Microscopy of a 'wet mount' with fresh, unstained blood on a slide under a coverslip can provide a diagnosis within minutes. Microscopy of blood smears remains important in the diagnosis of tropical febrile diseases, not only for quantifying parasitaemia and verifying the species in malaria, but also for revealing potentially deadly infections such as African sleeping sickness, babesiosis and relapsing fevers caused by Borrelia duttoni and B. recurrentis. A simple card agglutination test for trypanosomiasis (CATT) is used to screen for T.b. gambiense in high-risk areas, and rapid testing for T.b. gambiense has recently been introduced (10). The parasite can also be detected by nucleic acid-based tests such as PCR, if available (3).
The difference in treatment for T.b. gambiense and T.b. rhodesiense is particularly problematic in Uganda, which is the only country where both subspecies are endemic (11). Our patient was infected with T.b. rhodesiense in Murchison Falls, western Uganda, and thus was at risk of receiving the wrong treatment for his infection.
African sleeping sickness is a classic neglected disease that affects poor areas, and there is consequently a lack of financial incentive for drug development (3). However, efforts by various stakeholders in recent years have led to new effective treatment options for T.b. gambiense infection, such as combination therapy with oral nifurtimox and intravenous eflornithine (NECT) for stage 2 disease (12), oral fexinidazole, which is now first-line treatment for both stages (13), and the promising new drug acoziborol, which is effective as a single oral dose for both stages (14). Safer treatment options need to be established for T.b. rhodesiense soon.
The patient's perspective
Dangerous flies in East Africa encounter a well-prepared Norwegian health service
Ouch, a cleg bit me hard on the hand. I found out it was a tsetse fly. A week later, I felt ill. My roommate took me to the doctor, who didn't take me seriously. I sought advice when I started to feel even worse but was asked to wait. My wife booked a flight for me, and my roommate helped me to the airport, where the 'flight from hell' awaited. I felt an incredible sense of relief as I sank into the hospital bed in Norway. I was now in good hands. The rest was all smooth sailing, despite how ill I had been. I feel incredibly privileged because I was able to go home and get the care I needed.
Summary
African sleeping sickness is a major public health issue in parts of Africa and in rare cases can affect safari tourists and visitors. Our patient had an acute, life-threatening infection with T.b. rhodesiense that required treatment with special medication that few Norwegian hospitals stock. Diagnosis was made through microscopy of blood smears.
The patient has consented to publication of the article.
The article has been peer-reviewed.
- 1.
Camprubí-Ferrer D, Oteo JA, Bottieau E et al. Doxycycline responding illnesses in returning travellers with undifferentiated non-malaria fever: a European multicentre prospective cohort study. J Travel Med 2023; 30. doi: 10.1093/jtm/taac094. [PubMed][CrossRef]
- 2.
Crump JA, Morrissey AB, Nicholson WL et al. Etiology of severe non-malaria febrile illness in Northern Tanzania: a prospective cohort study. PLoS Negl Trop Dis 2013; 7. doi: 10.1371/journal.pntd.0002324. [PubMed][CrossRef]
- 3.
Büscher P, Cecchi G, Jamonneau V et al. Human African trypanosomiasis. Lancet 2017; 390: 2397–409. [PubMed][CrossRef]
- 4.
Jamonneau V, Ilboudo H, Kaboré J et al. Untreated human infections by Trypanosoma brucei gambiense are not 100% fatal. PLoS Negl Trop Dis 2012; 6. doi: 10.1371/journal.pntd.0001691. [PubMed][CrossRef]
- 5.
Schmid C, Richer M, Bilenge CM et al. Effectiveness of a 10-day melarsoprol schedule for the treatment of late-stage human African trypanosomiasis: confirmation from a multinational study (IMPAMEL II). J Infect Dis 2005; 191: 1922–31. [PubMed][CrossRef]
- 6.
Pepin J, Milord F, Guern C et al. Trial of prednisolone for prevention of melarsoprol-induced encephalopathy in gambiense sleeping sickness. Lancet 1989; 1: 1246–50. [PubMed][CrossRef]
- 7.
Blum J, Schmid C, Burri C. Clinical aspects of 2541 patients with second stage human African trypanosomiasis. Acta Trop 2006; 97: 55–64. [PubMed][CrossRef]
- 8.
Kuepfer I, Hhary EP, Allan M et al. Clinical presentation of T.b. rhodesiense sleeping sickness in second stage patients from Tanzania and Uganda. PLoS Negl Trop Dis 2011; 5. doi: 10.1371/journal.pntd.0000968. [PubMed][CrossRef]
- 9.
Migchelsen SJ, Büscher P, Hoepelman AI et al. Human African trypanosomiasis: a review of non-endemic cases in the past 20 years. Int J Infect Dis 2011; 15: e517–24. [PubMed][CrossRef]
- 10.
Büscher P, Mertens P, Leclipteux T et al. Sensitivity and specificity of HAT Sero-K-SeT, a rapid diagnostic test for serodiagnosis of sleeping sickness caused by Trypanosoma brucei gambiense: a case-control study. Lancet Glob Health 2014; 2: e359–63. [PubMed][CrossRef]
- 11.
Picozzi K, Fèvre EM, Odiit M et al. Sleeping sickness in Uganda: a thin line between two fatal diseases. BMJ 2005; 331: 1238–41. [PubMed][CrossRef]
- 12.
Priotto G, Kasparian S, Mutombo W et al. Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: a multicentre, randomised, phase III, non-inferiority trial. Lancet 2009; 374: 56–64. [PubMed][CrossRef]
- 13.
Mesu VKBK, Kalonji WM, Bardonneau C et al. Oral fexinidazole for late-stage African Trypanosoma brucei gambiense trypanosomiasis: a pivotal multicentre, randomised, non-inferiority trial. Lancet 2018; 391: 144–54. [PubMed][CrossRef]
- 14.
Betu Kumeso VK, Kalonji WM, Rembry S et al. Efficacy and safety of acoziborole in patients with human African trypanosomiasis caused by Trypanosoma brucei gambiense: a multicentre, open-label, single-arm, phase 2/3 trial. Lancet Infect Dis 2023; 23: 463–70. [PubMed][CrossRef]