MAIN FINDINGS
The number of COVID-19 symptoms/findings showed no clear association with antibody levels.
A total of 106/226 (47 %) COVID-19 convalescent plasma donors had ACE2 inhibitory antibody levels above the 60 % threshold or a positive SARS-CoV-2 neutralisation test.
Only standardised measurement of SARS-CoV-2 antibodies was suitable for selecting convalescent plasma donors in the early phase of the pandemic.
Convalescent plasma for the treatment of COVID-19 is a blood component from a donor who has recovered from COVID-19. Despite uncertainty about its efficacy, many countries, including Norway, initiated the collection and use of COVID-19 convalescent plasma. The set-up and background of the Norwegian project (NORPLASMA) is described in another article (1). In this study, we describe the donor material. Patient findings are published separately (2).
The varying course of the pandemic across countries meant that international data comparisons were crucial to providing better information about the optimal time for collecting plasma and the significance of testing for antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (3, 4). Individual variations in the amount of SARS-CoV-2 antibodies make standardised dosing of convalescent plasma challenging.
The main objective of the project was to produce potentially therapeutic plasma for patient treatment, but we also collected information to determine whether COVID-19 symptoms/findings could help identify donors with high antibody levels.
Material and method
A total of 448 donors from 12 blood banks across all Norwegian health regions consented to participate in the plasma donor project. Health information and blood samples for the research biobank were collected from 270 donors (1) (Figure 1) in the period 15 April 2020–1 July 2021. Plasma was collected from 266 donors, either by plasmapheresis, as an additional product during platelet apheresis, or from whole blood. The plasma collected was then frozen in units of 200–300 mL. Donors from the blood banks at Akershus, Haukeland, St. Olav's and North Norway university hospitals were included after antibody tests had been carried out, whereas at other blood banks, antibody testing was performed during the first plasma collection (1).
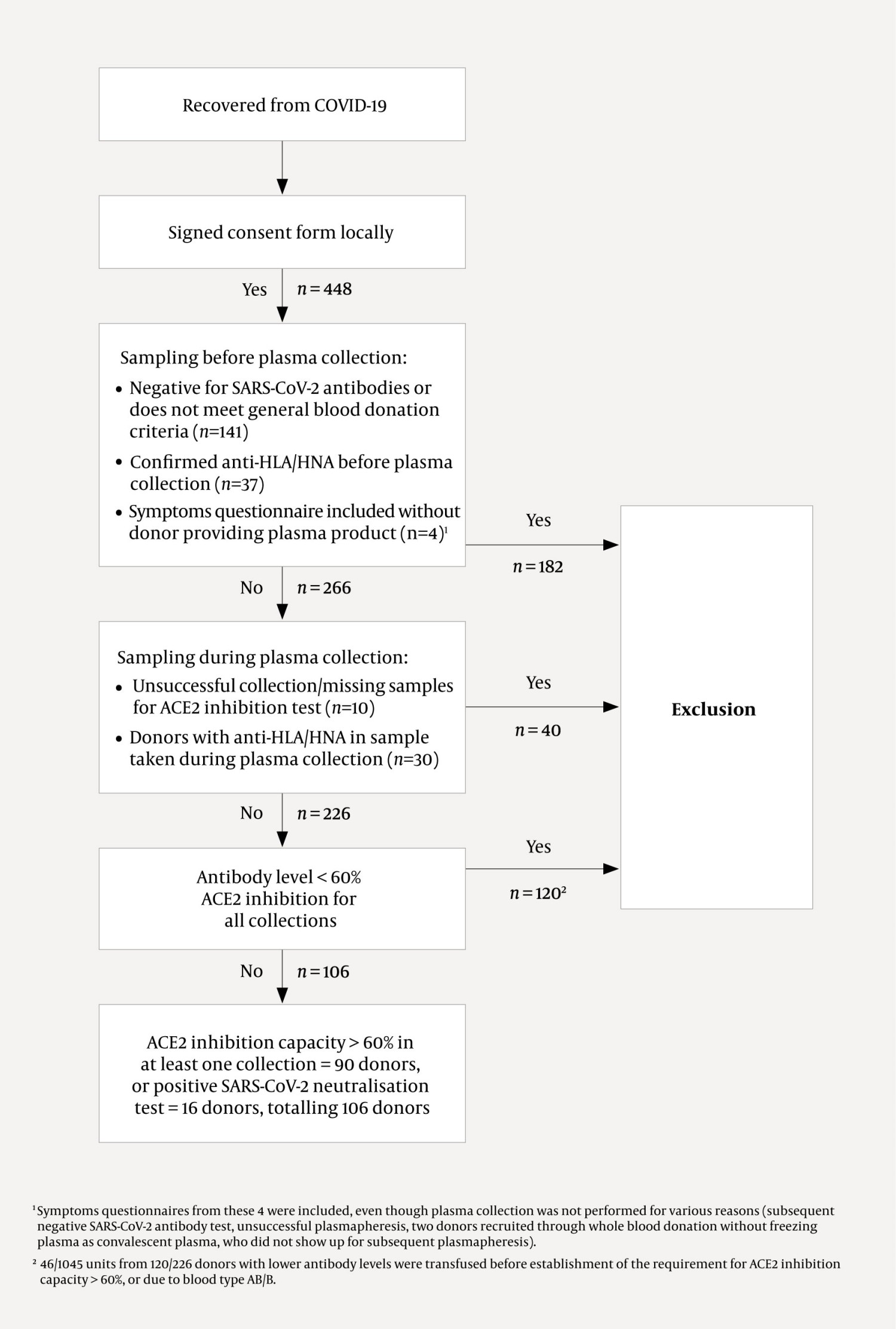
The donors completed a questionnaire that included questions on the timing of any polymerase chain reaction test (PCR test), hospitalisation and the presence of ten different COVID-19 symptoms/findings (Yes/No; n = 265). The list of symptoms (Table 1) is partly based on protocols from clinical studies (3, 5). A disease score was defined as the sum of the number of symptoms/findings and hospitalisation reported in the questionnaire, with possible values 0 (no symptoms/findings) to 11 (all reported symptoms/findings and hospitalisation).
Table 1
Demographics and disease scores for 270 donors of COVID-19 convalescent plasma at 12 blood banks in the period 15 April 2020–1 July 2021. Number (%) unless otherwise specified.
| Variables | Value | ||
|---|---|---|---|
| Sex |
| ||
|
| Female | 127 (47) | |
|
| Male | 143 (53) | |
| Age, years, median (interquartile range) | 44 (32–53) | ||
|
| Female | 42 (29–52) | |
|
| Male | 47 (35–53) | |
| Donors who reported symptoms/findings1 |
| ||
|
| Common cold symptoms | 198 (75) | |
|
| Fever | 194 (73) | |
|
| Mild cough | 188 (71) | |
|
| Impaired sense of taste/smell | 185 (70) | |
|
| Sore throat | 136 (51) | |
|
| Shortness of breath (dyspnoea) | 125 (47) | |
|
| Worsening of cough | 80 (30) | |
|
| Diarrhoea | 68 (26) | |
|
| Abdominal pain | 43 (16) | |
|
| Confirmed pneumonia | 5 (1,9)2 | |
| Donors who reported hospitalisation |
| ||
|
| Admitted to hospital | 12 (4,5) | |
|
|
| Admitted to intensive care unit | 3 (1,1) |
|
| Mechanical ventilation | 0 (0) | |
| No. of symptoms/findings3 |
| ||
|
| Median (interquartile range) | 5 (3–6) | |
1265 completed the symptoms questionnaire. Eight donors reported no symptoms.
2Two of whom were also hospitalised.
3The total number of self-reported symptoms/findings and hospitalisation, score 0–11.
Antibodies against SARS-CoV-2 were first detected using various locally established methods. Samples from plasma collected from 210/266 donors were later analysed at Oslo University Hospital to determine the inhibition of virus binding to angiotensin-converting enzyme 2 (ACE2). Calculated inhibition of viral ACE2 binding has been shown to correlate with SARS-CoV-2 neutralisation capacity (6). An antibody level that resulted in 60 % inhibition of ACE2 binding corresponded to a neutralisation titre of 1:100, and the antibody inhibition threshold to release the units for clinical use was therefore set at 60 % (1, 5). Plasma from 16 donors in the Central Norway Health Region was included based on SARS-CoV-2 neutralisation testing at St Olav's Hospital, without analysis of ACE2 binding at Oslo University Hospital. Unused plasma was sold for fractionation and technical use, or discarded (1).
Antibodies against human leukocyte/neutrophil antigens (HLA/HNA) were examined in LABScreen/LABScreen Multi bead-based assays (One Lambda) at Oslo University Hospital or the National Treatment Service for Advanced Platelet Immunology at the University Hospital of North Norway.
Statistics
Ledidi PRJCTS (Ledidi.com, Oslo) and Microsoft Office Excel 2016 were used for the collection and primary analysis of results. Descriptive statistics and testing for normal distribution were performed using GraphPad Prism version 9.4.1. Continuous data are reported as median (interquartile range) or mean (standard deviation (SD)).
Ethics
The project was approved by the Regional Committee for Medical and Health Research Ethics (REC) South-East (reference no. 140845) and the data protection officers at the participating blood banks. Personal data were stored and processed on the basis of consent, and approved procedures at Oslo University Hospital were followed.
Results
Demographic data from 270 blood donors approved for plasma donation are presented in Figure 1, and disease scores for 265 of these donors are shown in Table 1. Of the total donors, 143 (53 %) were men with a median age of 47 years (35–53). The median age for women was 42 years (29–52). The median disease score was 5 (3–6) (Table 1). The combination of cough, fever and shortness of breath was reported by 89/265 (34 %) donors, of which 53 (60 %) were men. Five donors reported confirmed pneumonia, and 12 donors had been hospitalised.
In total, 266 plasma donors from 12 blood banks contributed to the collection of 1644 units of plasma (Figure 1). Among these, 599/1644 plasma units obtained from 106/266 donors met the criterion of ACE2 inhibition capacity > 60 % (n = 90) or positive neutralisation test (n = 16), and were released for patient treatment (Figure 1). For 120/226 (53 %) donors, the ACE2 inhibition capacity was < 60 % (Figure 1, Figure 2). However, 46 plasma units with a lower antibody content were nevertheless transfused from these donors (2), either before the required ACE2 inhibition capacity was established (1) or in cases of the rare blood types AB or B.
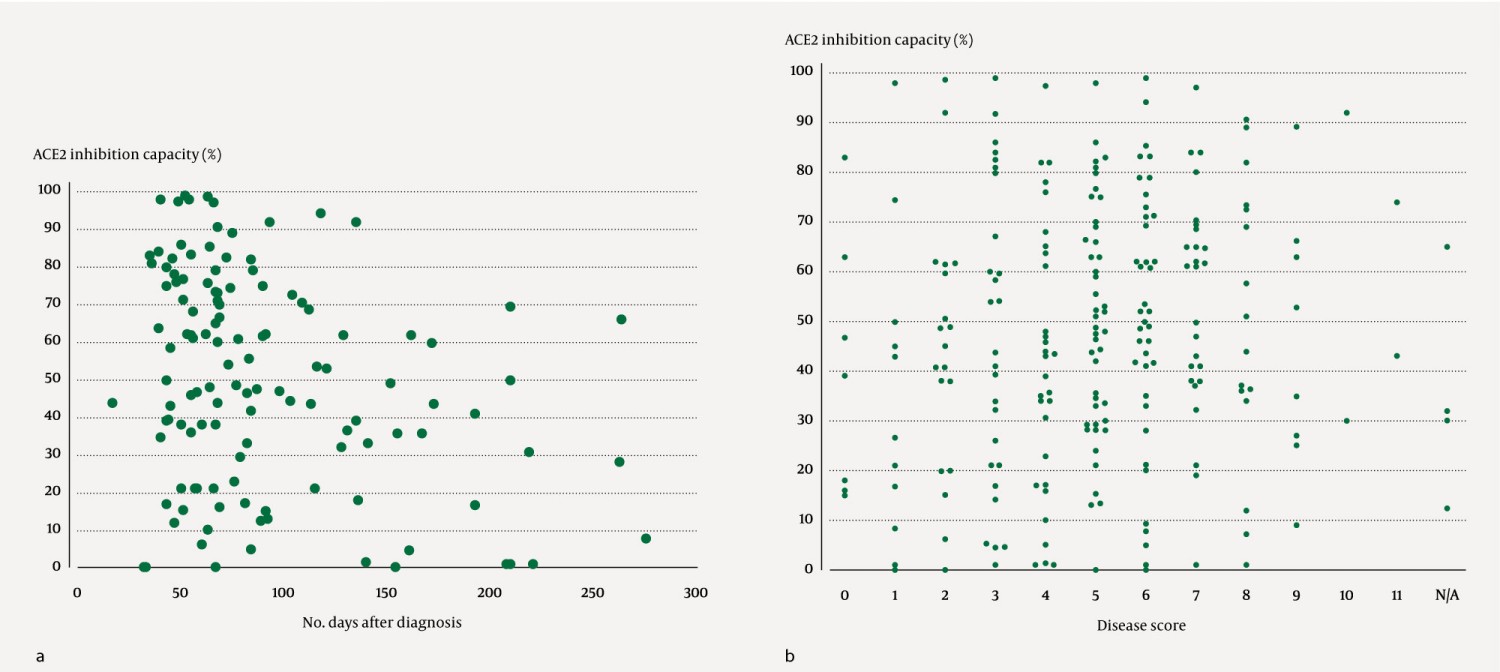
The antibody response after COVID-19 appeared to decrease over time (Figure 2a). The disease score appeared to be independent of ACE2 inhibition capacity (Figure 2b).
Tissue-type or leukocyte antibodies were confirmed in a total of 67/448 (15 %) of consenting donors. Plasma containing such antibodies, collected from 30/266 (11 %) donors, was not used to treat patients (Figure 1).
Discussion
Among plasma donors who met the criteria for convalescent plasma, we found a higher proportion of men compared to the general blood donor population, and they were older than the women. This has been reported in similar studies (7) and can be attributed to the selection of male donors (larger plasma volume, lower probability of tissue-type/leukocyte antibodies) and the fact that men were more severely affected by COVID-19 (8).
The initial reports on symptoms and antibody levels in COVID-19 patients showed a possible association between disease severity and titres of neutralising antibodies (3), while the type of symptom had less significance (3, 5). We therefore used the disease score as an expression of the severity of COVID-19 in this study. The lack of grading in symptom perception and severity is nevertheless a weakness of the study.
The proportion of donors with pneumonia and/or who were hospitalised was low, which is consistent with the generally good health of blood donors (9).
The antibody response varied considerably, despite the selected donor population. Only around 40 % of the collected plasma met the antibody content requirement of a 60 % ACE2 inhibition capacity, in line with published data (4). Another study found a correlation between the number of symptoms and antibody levels (3). We did not identify reliable markers for the development of antibodies with an ACE2 inhibition capacity > 60 %. It was not therefore possible to identify suitable convalescent plasma donors at an early stage of the project. However, rapidly decreasing ACE2 inhibitory antibody levels indicated that it was important to start the collection at an early stage. The study highlights how the quality of antibody testing is crucial for providing convalescent plasma for the purpose of patient treatment. Method development must be prioritised in future pandemics in order to minimise the collection of surplus plasma.
Specialist expertise, collaboration and flexibility enable rapid adaptation of blood components to new patient groups. The ability of Norwegian blood banks to unite quickly and efficiently to work on the project, largely within ordinary operations, could prove useful if a new situation requiring an escalation of national preparedness were to arise.
We would like to extend our thanks to everyone who made this project possible despite the pandemic and restrictions, especially the blood donors.
This article has been peer-reviewed.
- 1.
Nissen-Meyer LSH, Hervig T, Fevang B et al. Covid-19-rekonvalesensplasma fra norske blodgivere. Tidsskr Nor Legeforen 2022; 142: 768–70.
- 2.
Nissen-Meyer LSH, Macpherson ME, Skeie LG et al. Covid-19-pasienter behandlet med rekonvalesensplasma. Tidsskr Nor Legeforen 2023; 143. doi: 10.4045/tidsskr.22.0577. [CrossRef]
- 3.
Körper S, Jahrsdörfer B, Corman VM et al. Donors for SARS-CoV-2 Convalescent Plasma for a Controlled Clinical Trial: Donor Characteristics, Content and Time Course of SARS-CoV-2 Neutralizing Antibodies. Transfus Med Hemother 2021; 48: 137–47. [PubMed][CrossRef]
- 4.
Harvala H, Mehew J, Robb ML et al. Convalescent plasma treatment for SARS-CoV-2 infection: analysis of the first 436 donors in England, 22 April to 12 May 2020. Euro Surveill 2020; 25: 2001260. [PubMed][CrossRef]
- 5.
Lewin A, Therrien R, De Serres G et al. SARS-CoV-2 seroprevalence among blood donors in Québec, and analysis of symptoms associated with seropositivity: a nested case-control study. Can J Public Health 2021; 112: 576–86. [PubMed][CrossRef]
- 6.
Tran TT, Vaage EB, Mehta A et al. Titers of antibodies against ancestral SARS-CoV-2 correlate with levels of neutralizing antibodies to multiple variants. NPJ Vaccines 2022; 7: 174. [PubMed][CrossRef]
- 7.
Lasky B, Goodhue Meyer E, Steele WR et al. COVID-19 convalescent plasma donor characteristics, product disposition, and comparison with standard apheresis donors. Transfusion 2021; 61: 1471–8. [PubMed][CrossRef]
- 8.
FHI. COVID-19-epidemien. Risiko, prognose og respons i Norge. https://www.fhi.no/contentassets/c9e459cd7cc24991810a0d28d7803bd0/vedlegg/2020.05.19-notat-om-risiko-og-respons.pdf Accessed 11.1.2023.
- 9.
Mousavi SA, Hermundstad B, Saether PC et al. Health Behavior and Lifestyle Trends among Platelet Donors: Results from a Questionnaire-Based Survey in Norway. BioMed Res Int 2021; 2021: 8891885. [PubMed][CrossRef]