Three out of five members of a family developed COVID-19 following a trip abroad. A variety of serological assays detected SARS-CoV-2 antibodies – including neutralising antibodies – in all three individuals 3–4 weeks after symptom onset. Tests from one supplier were also positive in a fourth family member, probably owing to cross-reactivity. Robust serological assays are essential for determining prior infection and for identifying individuals who can donate plasma for use in COVID-19 therapy.
A family comprising a mother, father and their three adult children went on a week-long skiing holiday in Southern Europe. They were all in good health apart from one of the sons (son 2), who had influenza-like symptoms with fever, sore throat, rhinorrhoea and a mild cough prior to departure and during the first few days of the holiday. The parents stayed in one hotel room and the siblings in another. Two days before returning home, the daughter also became ill with reduced general condition, vomiting and a sore throat. She then gradually developed a subjective fever, rhinorrhoea, anosmia and headache. Owing to widespread COVID-19 in the area in which they had been staying, upon returning to Norway the parents and daughter immediately isolated themselves in the same residence. Two days after their return home, a nasopharyngeal specimen from the daughter was tested for SARS-CoV-2 RNA using polymerase chain reaction (PCR), but the result was negative. She developed a dry cough, and another test three days later was positive. The family's medical history is shown in Figure 1.
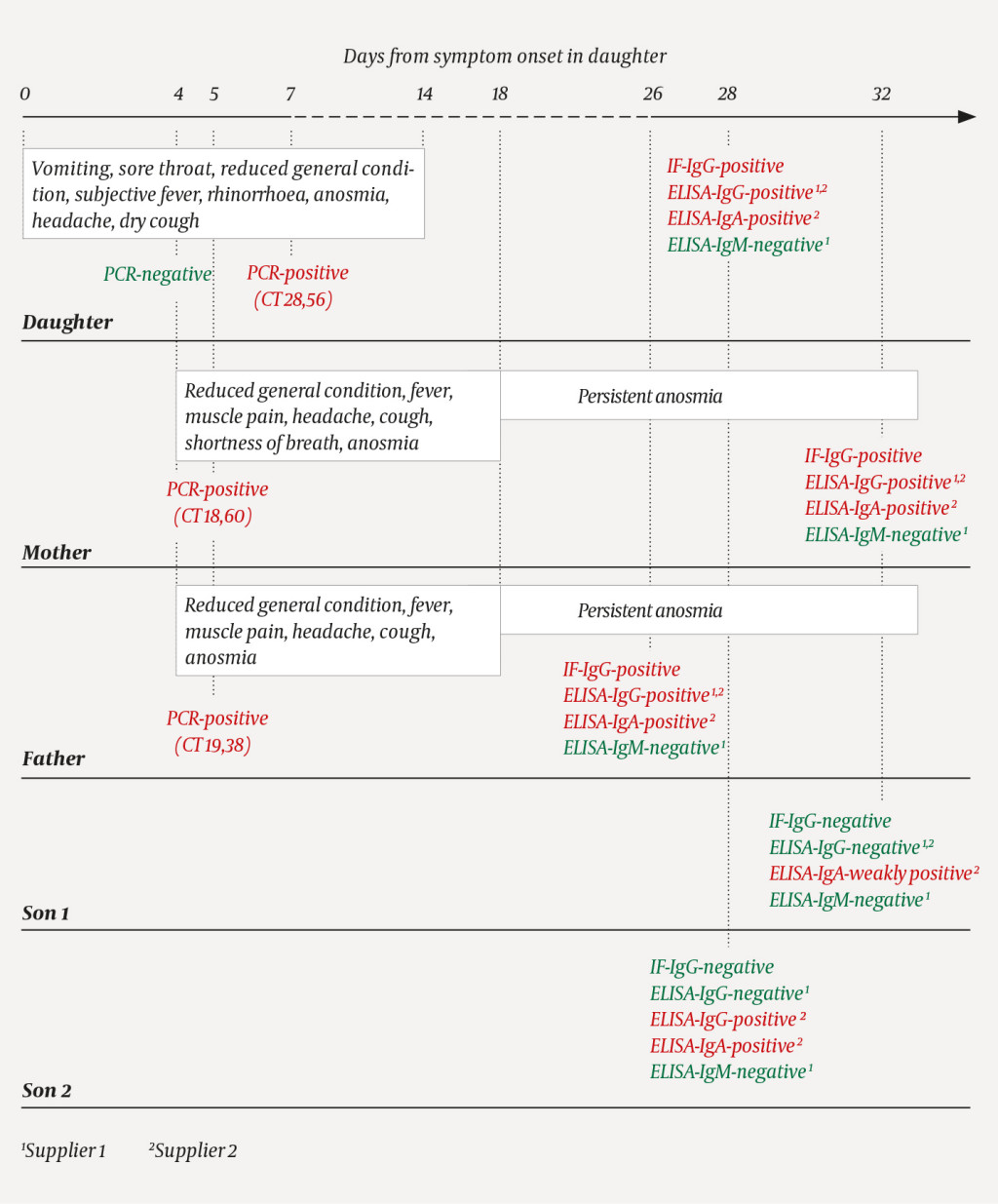
Two days after returning home, both parents also began to show reduced general condition with fever, muscle pain, headache, cough and eventually anosmia. The following day, both tested positive for SARS-CoV-2 RNA. Six days after symptom onset, the mother experienced transient shortness of breath and a deterioration in her general condition. While the daughter became symptom-free after two weeks, both parents still had slight anosmia six weeks after symptom onset. The two sons had no symptoms of COVID-19 and were not tested, but remained in home quarantine after their return to Norway.
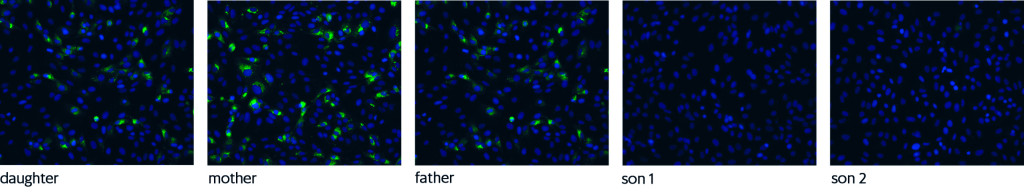
Blood samples from the family were included as part of a validation panel comprising a total of 46 sera. Three previously described serological assays were used Box 1) ((1), with minor modifications. Indirect immunofluorescence staining showed that serum from the parents and the daughter, but not from the sons, contained SARS-CoV-2-specific IgG (Figure 2). IgG Enzyme-Linked ImmunoSorbent Assay (IgG ELISA) tests from two different suppliers confirmed the presence of IgG in the parents and daughter, but the assay from supplier 2 also detected IgG in son 2. An IgA ELISA from supplier 2 detected SARS-CoV-2-specific IgA in all five individuals, but the IgA level in son 1 was very low. An IgM ELISA from supplier 1 failed to detect SARS-CoV-2-specific IgM in any family member. Only the three PCR-positive family members had detectable neutralising antibodies.
Indirect immunofluorescence staining reveals the presence of SARS-CoV-2-specific IgG directed against different viral proteins (antigens) in infected cells. Kidney cells from monkeys (Vero cells) are infected with SARS-CoV-2 and fixed the next day. Patient serum is added. Any antibodies against the SARS-CoV-2 antigen will bind to the antigen in infected cells. The addition of a fluorescent antibody against IgG will then make the infected cells fluoresce.
Indirect ELISA (enzyme-linked immunosorbent assay) detects the presence of SARS-CoV-2 specific antibodies directed against a selected viral antigen. Patient serum is added to a plastic well with antigen attached to the bottom. Any antibodies against the antigen will bind to it. An enzyme-labelled antibody against IgM, IgG or IgA is then added and binds to the bound antibody. The addition of substrate produces a measurable colour change.
Neutralisation tests are the gold standard for detecting SARS-CoV-2-specific neutralising antibodies of various immunoglobulin classes. Patient serum is serially diluted, SARS-CoV-2 is added, and the serum is then used to infect Vero cells. After fixation and indirect immunofluorescence staining with antibodies against SARS-CoV-2, the number of infected cells is estimated. The neutralisation titre is the dilution that reduces the number of infected cells by 50 %.
Discussion
It remains unclear which member of the family was the index patient. As son 2 developed symptoms prior to departure, at a time of very low SARS-CoV-2 transmission in Norway, it is unlikely that he was the index patient. The daughter developed symptoms while on holiday, but might have had a different infection initially. She had rhinorrhoea, which is not particularly common in cases of COVID-19, her first nasopharyngeal specimen collected four days after symptom onset was negative for SARS-CoV-2 RNA, and she apparently did not infect her brothers despite being in close proximity to them. Although virus secretion is greatest in most individuals in the upper respiratory tract upon symptom onset (2), there have been reports of patients who initially test negative and who have a fluctuating viral load (3). In addition to a low viral load, other factors that may cause a negative PCR result are poor specimen-collection technique, a long transport time, or laboratory errors. Moreover, it is not uncommon for only some members of a family to contract COVID-19. In a study of 105 families with COVID-19, 16.3 % of household members were found to be infected, with partners most at risk (4). Since both parents had positive PCR tests before their daughter, they may have been the index patients. We also cannot rule out the possibility that all three were infected by sources at the holiday destination.
Antibodies play a central role in the immune response to a viral infection. In the three family members with PCR-confirmed COVID-19, we were able to detect SARS-CoV-2-specific IgG, IgA and neutralising antibodies. We were unable to detect SARS-CoV-2-specific IgM, which is interesting as IgM is usually detected before IgG. People with mild symptoms have been reported to have lower SARS-CoV-2 antibody levels than those with severe disease (5), and this may have contributed to the negative results. IgA ELISA gave positive results for all family members, but son 1 was only weakly positive. IgA has an important role in influenza infections and is therefore also believed to play a role in COVID-19. The bulk of IgA is not found in serum, which is the material we test, but in the upper respiratory tract mucosa. The IgA ELISA test that we used is reported to have high sensitivity but low specificity (5), and we therefore believe that the positive IgA results for both sons were the result of non-specific reactivity. The IgG ELISA from supplier 2 was positive for son 2. The supplier has stated that the ELISA assays can detect antibodies against influenza virus, and cross-reactivity with antibodies against human coronavirus has been reported (5). The influenza-like illness in son 2 may therefore have contributed to false positive results for IgA and IgG. However, asymptomatic COVID-19 cannot be ruled out (2).
What do these results mean for the family? Positive ELISA and immunofluorescence staining reveal antigen-binding antibodies and confirm prior infection. However, on the basis of experience with other viruses, including SARS-CoV-1, detection of neutralising antibodies is the best indicator of protective immunity. It is still too early to say how long this immunity will last. Despite a lack of clinical trials, several COVID-19 patients have already been treated with plasma containing neutralising antibodies (6).
SARS-CoV-2 was discovered as recently as January 2020, and the manufacturers of diagnostic tests for COVID-19 have developed their assays in record time. Our experience indicates that not all commercial serological assays available for the diagnosis of COVID-19 are sufficiently robust.
The patients have consented to the publication of the article.
The article has been peer-reviewed.
- 1.
Kohmer N, Westhaus S, Rühl C et al. Clinical performance of different SARS-CoV-2 IgG antibody tests. J Med Virol 2020; 92: jmv.26145. [PubMed][CrossRef]
- 2.
Eurosurveillance Editorial Team. Updated rapid risk assessment from ECDC on coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK. Euro Surveill 2020; 25: 2003121.
- 3.
Li Y, Yao L, Li J et al. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J Med Virol 2020; 92: 903–8. [PubMed][CrossRef]
- 4.
Li W, Zhang B, Lu J et al. The characteristics of household transmission of COVID-19. Clin Infect Dis 2020; 71: ciaa450. [PubMed][CrossRef]
- 5.
Okba NMA, Müller MA, Li W et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease 2019 patients. Emerg Infect Dis 2020; 26: 1478–88. [PubMed][CrossRef]
- 6.
Rajendran K, Krishnasamy N, Rangarajan J et al. Convalescent plasma transfusion for the treatment of COVID-19: Systematic review. J Med Virol 2020; 92: jmv.25961. [PubMed][CrossRef]
Det skrives at test kjøres gjennom 40 sykluser. Så skrives det at CT var 18,5, og at dette er antall sykluser som ville vært nødvendig for å få positivt prøvesvar.
Har jeg forstått det rett? Kjøres uansett alle prøver gjennom minimum 40 sykluser? Stemmer det ikke at PCR-tester er ansett som svært upålitelig når sykluser overskrider 35? At alt over 35 sykluser vil føre til en oversensitiv test? Den vil kunne gi positive svar ved for små spor av blant annet korona til at det utvikles sykdom og med det smittespredning.
Ved CT 18, ble det kjørt en test på 20 sykluser for å sjekke om den ga positivt resultat?
Vi takker Herland for kommentaren til artikkelen Antistoffrespons hos en familie med covid-19, som gir oss mulighet til å oppklare betydningen av syklusterskel (cycle threshold, CT) ved polymerasekjedereaksjon (PCR).
Ved PCR er antall sykluser som kjøres avhengig av PCR-test, prøvemateriale og instrumentering. Vi benytter en SARS-CoV-2-spesifikk semikvantitativ PCR-test der alle prøvene blir kjørt i 40 sykluser før resultatene blir avlest. CT-verdi viser til den syklusen hvor man først påviser et signal som er signifikant høyere enn bakgrunnsstøy og verdien er omvendt proporsjonal med mengde virus-RNA i prøven.
Siden sensitiviteten til PCR-test øker med økt antall sykluser, øker også risiko for falske positive signaler. For å avdekke dette blir alle prøver analysert i duplikat og prøver med avvikende resultater kjøres på nytt. Alle prøver med CT-verdi over 37 svares inkonklusiv og det bes om nye prøver.
Funn av høy CT-verdi skyldes vanligvis at prøven er tatt svært tidlig eller sent i sykdomsforløpet. Det er riktig at en høy CT-verdi i prøver fra øvre luftvei tyder på at pasienten er lite smitteførende, men hvis prøven er tatt tidlig i forløpet kan virusmengde i pasienten være økende. I tillegg kan høy CT-verdi skyldes dårlig prøvekvalitet på grunn av prøvetakingsteknikk eller lang transportvei, eller at infeksjonsfokuset er anatomisk forskjellig fra prøvetakingsstedet. Prøver med høy CT-verdi bør derfor tolkes på bakgrunn av kliniske opplysninger. Alle positive prøver svares ut av lege.
For vår kasuistikk sammenfalt positive PCR-resultater med kliniske opplysninger og kunne senere bekreftes med serologiske tester. Påvisning av virus-RNA som ga CT-verdi på 18,6 hos mor og 19,38 hos far indikerte høy virusmengde i prøvemateriale, mens en CT-verdi på 28,56 hos datter indikerte middels virusmengde.