Myocardial fibrosis arises when the heart's fibroblasts produce collagenous scar tissue. This process may primarily be reparative, but over time often has negative consequences for the patient in the form of function impairment, morbidity and mortality. Cardiovascular disease is still the dominant cause of death in the Western world (1), and myocardial fibrosis arises in the course of many heart diseases. This review article first presents the pathophysiology and classification of myocardial fibrosis and methods for its detection. Some conditions in which myocardial fibrosis can be detected are then described, with the focus on aortic stenosis. Finally, future possibilities are described, with the focus on diagnostics and therapy.
Method
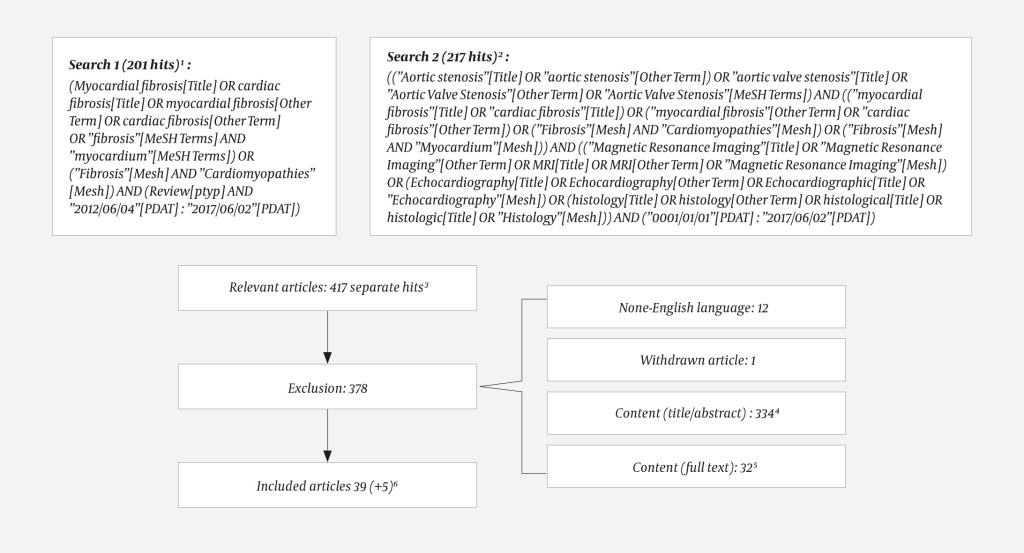
The article is based on two complementary literature searches conducted in PubMed on 2 June 2017. First, we searched for review articles from the last five years in which the terms "myocardial fibrosis" or "cardiac fibrosis" were used in the title, or occurred as MeSH terms (medical subject headings) or as other terms (author's tagging of article). In the second search, we combined searches for "aortic stenosis" "myocardial fibrosis" with detection methods (cardiac MRI, echocardiography or histology, with synonyms). Without defining time limits, we searched for the terms in the title, MeSH or other terms. The search details with number of hits, excluded and included articles, are illustrated in Figure 1. The first author excluded 378 of 417 hits on the basis of content. Five articles were added. A total of 44 articles were included, and all were read in full text.
Pathophysiology and classification
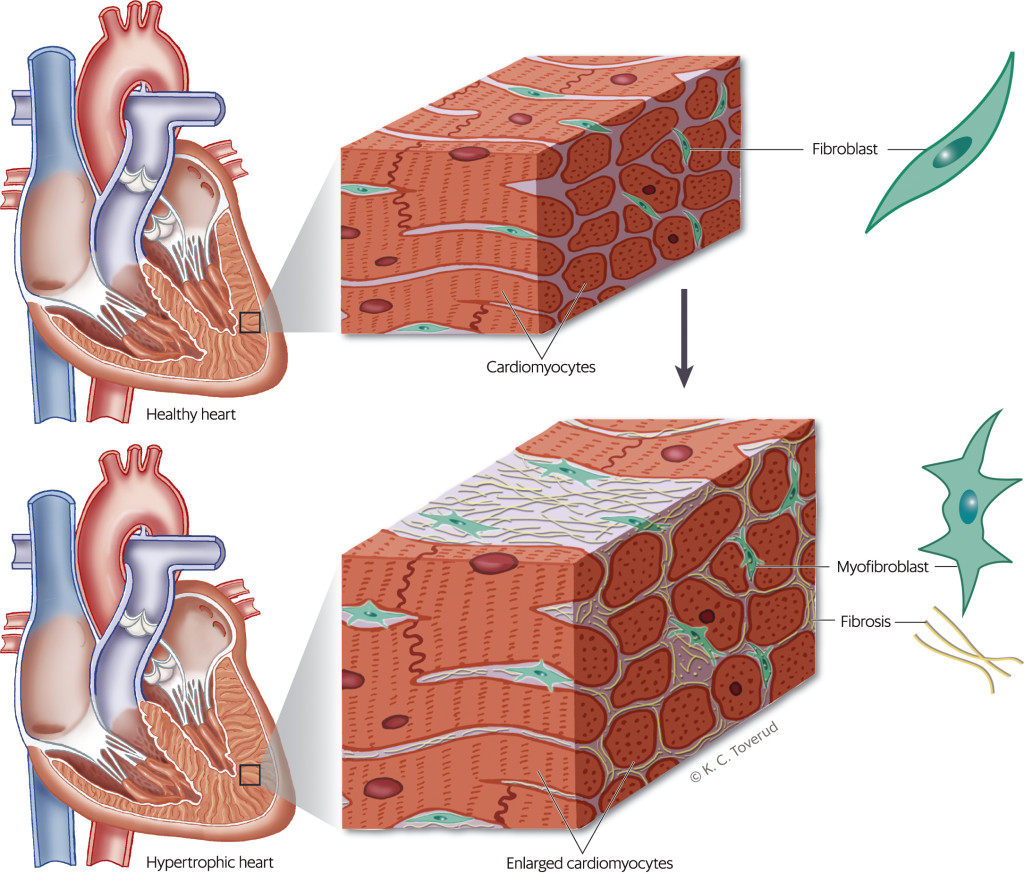
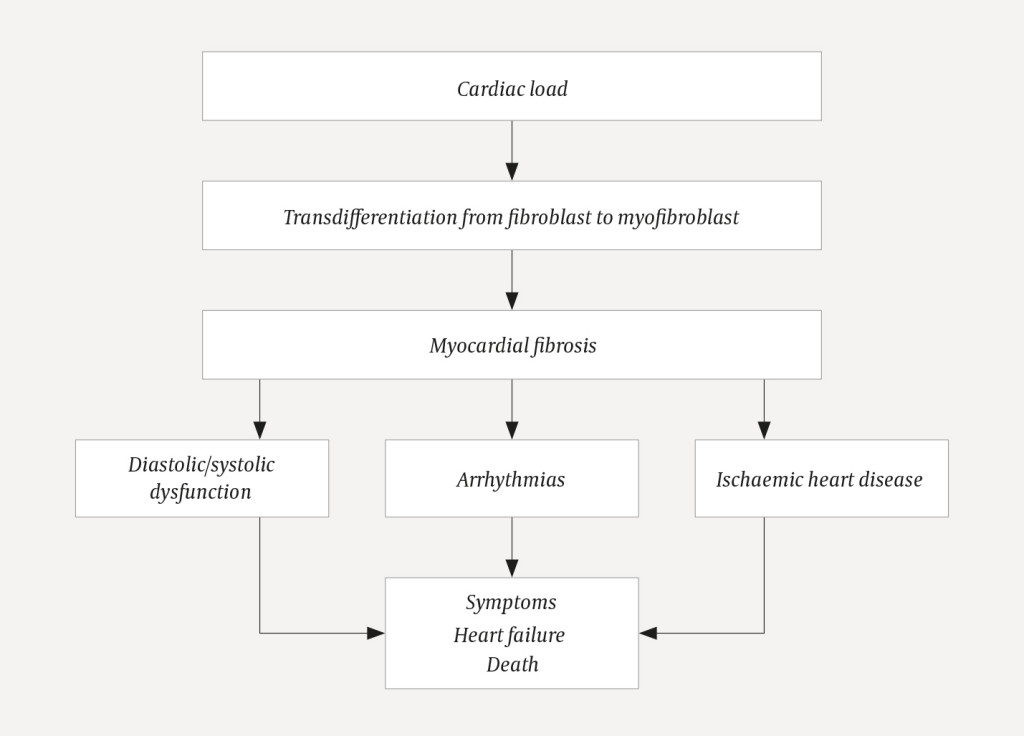
When cardiac damage and stress occur, various substances cause fibroblasts to become activated and transdifferentiate into myofibroblasts (2)−(7) (Figure 2) (8). The myofibroblasts increase the production of proteins that are deposited in the extracellular matrix (2–5). Collagen I, which accounts for about 80 % of the collagen in the myocardium, makes the myocardium stiffer and increases most in myocardial fibrosis (7, 9, 10). Cross-linking makes the collagen matrix stiffer and more difficult to break down with proteinases (2, 3, 5, 11). Fibrosis occurs as a result of net collagen production. Fibrosis restricts the supply of oxygen and nourishment to the myocardium (2, 3). Myocardial fibrosis causes electrical and structural changes that predispose patients to arrhythmias, heart failure and ischaemia (12). Figure 3 summarises the pathophysiology and consequences of myocardial fibrosis.
Fibrosis is classified by cause and pathoanatomy (3, 13). Interstitial (diffuse) fibrosis is characterised by diffuse spread of extracellular collagen without cardiomyocyte necrosis (8), and is believed to be reversible if early, focused treatment is administered (3, 7, 14). Diffuse fibrosis is seen in elderly people and in cardiac diseases such as aortic stenosis, cardiomyopathies and coronary heart disease without infarction (7, 15). Replacement fibrosis (scar fibrosis) is local and occurs after cardiomyocyte necrosis, for example after myocardial infarction. Replacement fibrosis is regarded as irreversible, and prevents cardiac muscle rupture after infarction (2–4), (4, 10, 13, 14, 16). However, it may also arise in the course of other diseases (3, 5, 7, 14, 16).
Detection of myocardial fibrosis
Biological specimen
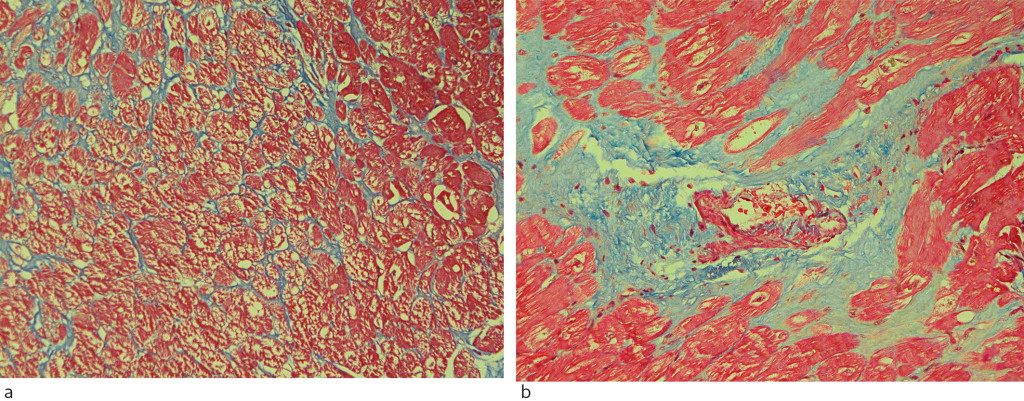
Biomarkers for fibrosis can be measured in myocardial biopsies and blood tests. Myocardial biopsies are taken from explanted hearts or during myectomy, open heart surgery or catheter-based endocardial biopsy. With heart biopsies and using appropriate staining methods, histological analysis of the volume fraction of collagen is regarded as the gold standard for detection of fibrosis (7, 10, 17). Total collagen can be quantified, and the type (intrastitial or replacement) and extent of fibrosis can be described (Figure 4). Limitations are associated with possible non-representative biopsy, purely local assessment, limited amount of tissue and procedure-related risk (15), (17–19). Modern molecular biology methods can also be used, but should be validated against histology prior to clinical use (18).
Blood biomarkers reflect cellular and molecular changes related to the quantity of fibrotic tissue (9). C-terminal propeptides of collagen I and N-terminal propeptides of collagen III are adequately validated (9), and there is optimism concerning their future clinical use. Galectin-3 stimulates the activation of myofibroblasts and the development of myocardial fibrosis (20), but the plasma concentration of galectin-3 also increases in renal disease and liver and lung fibrosis (11). Galectin-3 level is associated with mortality and a worsened prognosis in heart failure with reduced or preserved ejection fraction (3, 11).
Cardiac MRI scan
Cardiac MRI is non-invasive in the absence of contrast injection, and maps the heart's tissue composition and function (10).
T1 relaxometry can be performed quickly without injection of contrast medium, and can therefore be carried out independently of kidney function tests (10, 16, 21). The method provides information about oedema, fibrosis (extended T1 relaxation time) and deposition diseases (7, 10, 21), and is used clinically and in research of several cardiac muscle diseases (16, 22).
In replacement fibrosis, qualitative differences can be detected in late enhancement with gadolinium-based contrast mediums (10, 18). After myocardial infarction, increased late enhancement is seen, consistent with replacement fibrosis (Figure 5). In non-ischaemic cardiac muscle diseases, late enhancement is associated with increased hypertrophy and a worsened prognosis in several conditions (23, 24). It is important to be aware that the late enhancement method is unsuitable for assessing fibrosis in cases of diffuse fibrosis with a homogenous myocardium (15, 25).
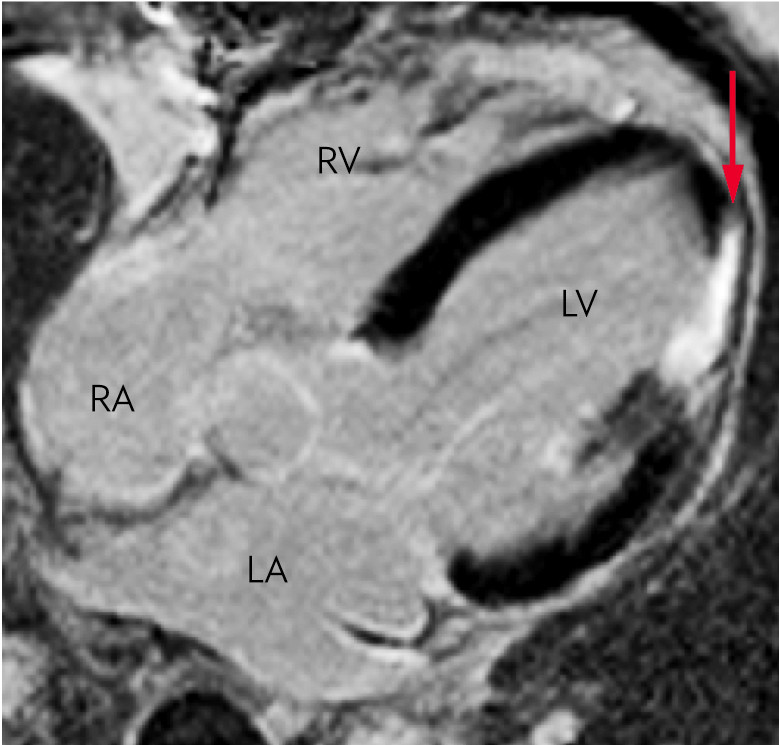
The heart's extracellular volume can be calculated from T1 relaxometry following gadolinium injection. In the absence of oedema or amyloid deposition, fibrosis will explain an increased extracellular volume (10, 26). The method can detect minor quantities of fibrosis and has shown better correlation with histological interstitial fibrosis than T1 relaxometry and conventional assessment of late enhancement (25). This means of calculating extracellular volume yields considerable prognostic information (10, 21). MRI determination of extracellular volume appears to be more myocardium-specific than blood biomarkers, and can be used for evaluating intervention efficacy (11).
Fibrosis as determined by MRI shows good correlation with histology (7, 10, 11, 15, 25). Heart MRI can be called a more feasible gold standard for detecting fibrosis (11, 26).
Cardiac CT scan
Scarring and other fibrosis can be detected very accurately with CT, but in the interests of radiation hygiene, it is seldom used (19). Assessment of fibrosis with cardiac CT has shown good correspondence with cardiac MRI methods and histology (17). The method is considered if MRI is contraindicated (17) and if there are other indications for cardiac CT (27).
Echocardiography
Diffuse fibrosis changes the wave propagation and reflection properties of the myocardium. These changes can be determined with the aid of ultrasound. Echocardiography provides additional information about the structure and function of the heart, and these parameters have been evaluated against MRI and histopathology with respect to fibrosis (6, 16). Echocardiography is cheap and readily available, but the assessment depends on image quality and operator experience (16, 18).
Table 1 sums up the strengths and weaknesses of the diagnostic methods in the detection of myocardial fibrosis.
Table 1
Advantages and drawbacks of different methods for detecting myocardial fibrosis. Scoring of suitability. The methods are compared on the basis of the authors' assessment.
| Histology |
Blood tests |
MRI |
CT |
Echocardiography |
|
|---|---|---|---|---|---|
| Direct detection of fibrosis 1 |
+++ |
− |
++(+) |
+ |
+ |
| Assessment of cardiac function |
− |
− |
+++ 2 |
++ |
+++ 2 |
| Resource-demanding (time/costs) |
+++ |
− |
++ |
+ |
+ |
| Availability |
− |
+++ |
+ |
++ |
++ |
| Risk of complications |
+(+) 3 |
(+) |
+ |
+ |
− |
| Radiation |
− 3 |
− |
− |
+++ |
− |
| Requires extensive training |
+++ |
+ |
++(+) |
++ |
+++ |
1Histology is the gold standard, while MRI is considered the non-invasive gold standard. The other methods are scored after validation against the gold standard.
2Almost as good as a gold standard for assessing cardiac function in relation to the consequences of the fibrosis.
3Presupposes tissue sampling during open heart surgery.
Myocardial fibrosis in various diseases
Myocardial fibrosis is associated with increased myocardial stiffness, cardiomyocyte necrosis, arrhythmias, sudden cardiac death and unfavourable prognosis (2, 3, 17, 18, 23, 28), and plays a central part in the remodelling process that leads to heart failure (10, 17, 26). Coronary heart disease, aortic stenosis and hypertension are the most frequent causes of myocardial fibrosis (13). Aortic stenosis and hypertension result in pressure overload of the left ventricle where the increased wall stress induces hypertrophy and interstitial fibrosis (2–4). Chronic pressure overload can cause cardiomyocyte necrosis and replacement fibrosis (22, 29).
Aortic stenosis
In aortic stenosis, histological fibrosis (interstitial and replacement) can constitute up to 30 % of the tissue volume (14). Both interstitial and replacement fibrosis can occur in the same individual. MRI studies show that interstitial fibrosis may predominate early in the course of the disease, while replacement fibrosis can be seen in up to 60 % of patients (14, 19). Fibrosis most frequently arises subendocardially and in the midwall, with greatest extension in the basal septum of the left ventricle (10, 28, 30). Local wall stress and increased wall thickness (with risk of ischaemia) may explain this.
In aortic stenosis, the extent of fibrosis is associated with symptom intensity, reduced cardiac function and physical capacity, and increased mortality (10, 22, 23, 28)(28–32). Extensive replacement fibrosis prior to valve surgery predicts an unfavourable post-operative outcome (23), (29–31), (33). Surgery for severe aortic stenosis is recommended if patients develop symptoms or a reduced ejection fraction, or simultaneously with other heart surgery (34). However, preoperative assessment has challenges (27). Valve surgery should be considered before irreversible fibrosis or function impairment occurs, as early intervention may improve the prognosis for asymptomatic patients with preserved ejection fraction (23, 29). In the future, diagnostic imaging, blood biomarkers and risk calculators may help to optimise patient selection and the timing of valve surgery (31, 33).
Hypertension
In hypertensive heart disease, interstitial fibrosis will translate into an unfavourable prognosis and contribute to cardiac dysfunction, coronary heart disease and arrhythmias (35). Cardiac MRI can predict future decompensation (10). The extent of fibrosis is limited in isolated hypertension, but increases with concomitant hypertrophy (36) or chronic kidney disease (16). Hypertensive patients can use inhibitors of the renin-angiotensin-aldosterone system with antifibrotic properties (36). Blood pressure reduction may cause regression of interstitial fibrosis.
Hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy is a genetic disease that can cause extensive myocardial fibrosis (37) (Figure 4). Fibrosis and function impairment are often greatest in the basal septum (38). High late contrast enhancement in MRI is associated with increased risk of sudden cardiac death (39). Interstitial fibrosis is more strongly associated with arrhythmias than replacement fibrosis (37).
Cardiac amyloidosis
Cardiac amyloidosis, also called infiltrating interstitial fibrosis (7, 16), involves deposition of amyloid in the myocardium. In contrast to traditional myocardial fibrosis, the condition arises independently of cardiac stress or load (11). In cardiac amyloidosis, ECG and imaging may yield findings that resemble myocardial fibrosis (low voltage, hypertrophy and signs of fibrosis). Various biomarkers and cardiac MRI can distinguish between the conditions. Cardiac amyloidosis causes severe heart failure with a poor prognosis (26).
Ischaemic heart disease and dilated cardiomyopathy
Non-ischaemic dilated cardiomyopathy and ischaemic heart disease are the two most frequent causes of heart failure and heart transplantation. An increased amount of replacement fibrosis, detected by MRI, is associated with a poor prognosis and severe arrhythmias (7). In dilated cardiomyopathy, fibrosis is frequently located in the midwall with patchy or diffuse distribution (7). Ischaemic replacement fibrosis occurs in transmural or subendocardial locations with regional extension corresponding to the anatomy of the coronary arteries, and can be detected by means of MRI (7). After a transmural myocardial infarction, the myocardium may appear akinetic, thinned and with higher echogenicity on an echocardiogram.
Atrial fibrillation
In cases of atrial fibrillation, an increased amount of atrial fibrosis is seen. Severe atrial fibrosis is associated with more frequent attacks of atrial fibrillation, embolic stroke and less efficacy from antiarrhythmic drugs and radio frequency ablation (6, 40, 41). Atrial fibrosis can to a certain extent be detected by means of echocardiography and MRI, where only MRI is validated against histology. (40, 41).
Other conditions
Fibrosis implies a poor prognosis in aortic regurgitation. One study found that the extent of fibrosis in severe aortic regurgitation resembles fibrosis in severe aortic stenosis (31).
Myocardial fibrosis is seen with severe kidney failure, particularly among patients undergoing haemodialysis treatment. Kidney transplant patients have less myocardial fibrosis than patients who undergo haemodialysis, and regression of myocardial fibrosis has been observed after transplantation (16).
Diabetic cardiomyopathy is characterised by early development of diastolic dysfunction, hypertrophy and diffuse fibrosis (42). Diabetics have higher extracellular volume judging by MRI with late enhancement, and are at increased risk of death or hospitalisation in the event of heart failure (26).
Physiological hypertrophy in connection with training and pregnancy is reversible and does not normally cause fibrosis (3). However, fibrosis can be encountered in older athletes. In cases of myocardial fibrosis in young athletes, the possibility of underlying cardiomyopathy should be considered (43).
Future therapeutic possibilities
Intensive research is in progress to develop medication that reverses or halts the development of myocardial fibrosis, as it is believed that this could revolutionise the treatment of heart failure (2, 4, 11). The two main strategies for reducing fibrosis are to inhibit profibrotic or to stimulate antifibrotic molecules (5). Transforming growth factor-β (TGF-β) and galectin-3 play a central part in the development of myocardial fibrosis (2, 4, 20), and fibrosis mediated by angiotensin II and aldosterone depends partly on these molecules (7, 44). Inhibition of TGF-β and galectin-3 has exhibited an antifibrotic effect (2, 44). Inhibitors of the renin-angiotensin-aldosterone system halt the development of fibrosis and can partly explain the efficacy of these drugs in cases of heart failure and hypertension (2, 4, 5, 11).
Prevention and effective treatment of established cardiac disease according to guidelines for limiting the development of fibrosis is important (45).
Conclusion
Modern biomarkers and diagnostic imaging have improved our understanding of myocardial fibrosis and its prognostic significance for several cardiac diseases. Improved methods for detection, an increased understanding of central molecular signalling pathways, and potential antifibrotic treatments are key areas of research (12). Assessment of myocardial fibrosis will probably gain a bigger place in the diagnosis, risk stratification and treatment of cardiac disease in the future.
Thanks to Katrine Aronsen at the University Library in Trondheim for her contributions to the literature search, and research fellow Erik Andreas Rye Berg at the Norwegian University of Science and Technology for valuable input.
Main points
Myocardial fibrosis is defined as an increased quantity of collagenous scar tissue in the heart
Myocardial fibrosis may arise as a result of cardiac disease and/or extracardiac diseases
Myocardial fibrosis is associated with an unfavourable prognosis
Modern diagnostics facilitate the detection of myocardial fibrosis
- 1.
Townsend N, Wilson L, Bhatnagar P et al. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J 2016; 37: 3232 - 45. [PubMed][CrossRef]
- 2.
Travers JG, Kamal FA, Robbins J et al. Cardiac fibrosis: the fibroblast awakens. Circ Res 2016; 118: 1021 - 40. [PubMed][CrossRef]
- 3.
Piek A, de Boer RA, Silljé HH. The fibrosis-cell death axis in heart failure. Heart Fail Rev 2016; 21: 199 - 211. [PubMed][CrossRef]
- 4.
Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci 2014; 71: 549 - 74. [PubMed][CrossRef]
- 5.
Talman V, Ruskoaho H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res 2016; 365: 563 - 81. [PubMed][CrossRef]
- 6.
Dzeshka MS, Lip GY, Snezhitskiy V et al. Cardiac fibrosis in patients with atrial fibrillation: mechanisms and clinical implications. J Am Coll Cardiol 2015; 66: 943 - 59. [PubMed][CrossRef]
- 7.
Barison A, Grigoratos C, Todiere G et al. Myocardial interstitial remodelling in non-ischaemic dilated cardiomyopathy: insights from cardiovascular magnetic resonance. Heart Fail Rev 2015; 20: 731 - 49. [PubMed][CrossRef]
- 8.
Herum KM, Lunde IG, McCulloch AD et al. The soft- and hard-heartedness of cardiac fibroblasts: mechanotransduction signaling pathways in fibrosis of the heart. J Clin Med 2017; 6: E53. [PubMed][CrossRef]
- 9.
López B, González A, Ravassa S et al. Circulating biomarkers of myocardial fibrosis: the need for a reappraisal. J Am Coll Cardiol 2015; 65: 2449 - 56. [PubMed][CrossRef]
- 10.
Everett RJ, Stirrat CG, Semple SI et al. Assessment of myocardial fibrosis with T1 mapping MRI. Clin Radiol 2016; 71: 768 - 78. [PubMed][CrossRef]
- 11.
Schelbert EB, Fonarow GC, Bonow RO et al. Therapeutic targets in heart failure: refocusing on the myocardial interstitium. J Am Coll Cardiol 2014; 63: 2188 - 98. [PubMed][CrossRef]
- 12.
Heymans S, González A, Pizard A et al. Searching for new mechanisms of myocardial fibrosis with diagnostic and/or therapeutic potential. Eur J Heart Fail 2015; 17: 764 - 71. [PubMed][CrossRef]
- 13.
Zeisberg M, Kalluri R. Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am J Physiol Cell Physiol 2013; 304: C216 - 25. [PubMed][CrossRef]
- 14.
Chin CW, Vassiliou V, Jenkins WS et al. Markers of left ventricular decompensation in aortic stenosis. Expert Rev Cardiovasc Ther 2014; 12: 901 - 12. [PubMed][CrossRef]
- 15.
Flett AS, Hayward MP, Ashworth MT et al. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation 2010; 122: 138 - 44. [PubMed][CrossRef]
- 16.
Graham-Brown MP, Patel AS, Stensel DJ et al. Imaging of myocardial fibrosis in patients with end-stage renal disease: current limitations and future possibilities. BioMed Res Int 2017; 2017: 5453606. [PubMed][CrossRef]
- 17.
Pattanayak P, Bleumke DA. Tissue characterization of the myocardium: state of the art characterization by magnetic resonance and computed tomography imaging. Radiol Clin North Am 2015; 53: 413 - 23. [PubMed][CrossRef]
- 18.
Sado DM, Flett AS, Moon JC. Novel imaging techniques for diffuse myocardial fibrosis. Future Cardiol 2011; 7: 643 - 50. [PubMed][CrossRef]
- 19.
Badiani S, van Zalen J, Treibel TA et al. Aortic stenosis, a left ventricular disease: insights from advanced imaging. Curr Cardiol Rep 2016; 18: 80. [PubMed][CrossRef]
- 20.
Hundae A, McCullough PA. Cardiac and renal fibrosis in chronic cardiorenal syndromes. Nephron Clin Pract 2014; 127: 106 - 12. [PubMed][CrossRef]
- 21.
Moon JC, Messroghli DR, Kellman P et al. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson 2013; 15: 92. [PubMed][CrossRef]
- 22.
Chin CWL, Everett RJ, Kwiecinski J et al. Myocardial fibrosis and cardiac decompensation in aortic stenosis. JACC Cardiovasc Imaging 2017; 10: 1320 - 33. [PubMed][CrossRef]
- 23.
Quarto C, Dweck MR, Murigu T et al. Late gadolinium enhancement as a potential marker of increased perioperative risk in aortic valve replacement. Interact Cardiovasc Thorac Surg 2012; 15: 45 - 50. [PubMed][CrossRef]
- 24.
Rudolph A, Abdel-Aty H, Bohl S et al. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J Am Coll Cardiol 2009; 53: 284 - 91. [PubMed][CrossRef]
- 25.
de Meester de Ravenstein C, Bouzin C, Lazam S et al. Histological Validation of measurement of diffuse interstitial myocardial fibrosis by myocardial extravascular volume fraction from Modified Look-Locker imaging (MOLLI) T1 mapping at 3 T. J Cardiovasc Magn Reson 2015; 17: 48. [PubMed][CrossRef]
- 26.
Bulluck H, Maestrini V, Rosmini S et al. Myocardial T1 mapping. Circ J 2015; 79: 487 - 94. [PubMed][CrossRef]
- 27.
Chin CW, Pawade TA, Newby DE et al. Risk stratification in patients with aortic stenosis using novel imaging approaches. Circ Cardiovasc Imaging 2015; 8: e003421. [PubMed][CrossRef]
- 28.
Dweck MR, Joshi S, Murigu T et al. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol 2011; 58: 1271 - 9. [PubMed][CrossRef]
- 29.
Milano AD, Faggian G, Dodonov M et al. Prognostic value of myocardial fibrosis in patients with severe aortic valve stenosis. J Thorac Cardiovasc Surg 2012; 144: 830 - 7. [PubMed][CrossRef]
- 30.
Weidemann F, Herrmann S, Störk S et al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation 2009; 120: 577 - 84. [PubMed][CrossRef]
- 31.
Azevedo CF, Nigri M, Higuchi ML et al. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol 2010; 56: 278 - 87. [PubMed][CrossRef]
- 32.
Flett AS, Sado DM, Quarta G et al. Diffuse myocardial fibrosis in severe aortic stenosis: an equilibrium contrast cardiovascular magnetic resonance study. Eur Heart J Cardiovasc Imaging 2012; 13: 819 - 26. [PubMed][CrossRef]
- 33.
Chin CW, Messika-Zeitoun D, Shah AS et al. A clinical risk score of myocardial fibrosis predicts adverse outcomes in aortic stenosis. Eur Heart J 2016; 37: 713 - 23. [PubMed][CrossRef]
- 34.
Baumgartner H, Falk V, Bax JJ et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017; 38: 2739 - 91. [PubMed][CrossRef]
- 35.
Moreno MU, Eiros R, Gavira JJ et al. The hypertensive myocardium: from microscopic lesions to clinical complications and outcomes. Med Clin North Am 2017; 101: 43 - 52. [PubMed][CrossRef]
- 36.
Treibel TA, Zemrak F, Sado DM et al. Extracellular volume quantification in isolated hypertension - changes at the detectable limits? J Cardiovasc Magn Reson 2015; 17: 74. [PubMed][CrossRef]
- 37.
Almaas VM, Haugaa KH, Strøm EH et al. Increased amount of interstitial fibrosis predicts ventricular arrhythmias, and is associated with reduced myocardial septal function in patients with obstructive hypertrophic cardiomyopathy. Europace 2013; 15: 1319 - 27. [PubMed][CrossRef]
- 38.
Doesch C, Sperb A, Sudarski S et al. Mitral annular plane systolic excursion is an easy tool for fibrosis detection by late gadolinium enhancement cardiovascular magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Arch Cardiovasc Dis 2015; 108: 356 - 66. [PubMed][CrossRef]
- 39.
Briasoulis A, Mallikethi-Reddy S, Palla M et al. Myocardial fibrosis on cardiac magnetic resonance and cardiac outcomes in hypertrophic cardiomyopathy: a meta-analysis. Heart 2015; 101: 1406 - 11. [PubMed][CrossRef]
- 40.
Hirsh BJ, Copeland-Halperin RS, Halperin JL. Fibrotic atrial cardiomyopathy, atrial fibrillation, and thromboembolism: mechanistic links and clinical inferences. J Am Coll Cardiol 2015; 65: 2239 - 51. [PubMed][CrossRef]
- 41.
Akoum N, Marrouche N. Assessment and impact of cardiac fibrosis on atrial fibrillation. Curr Cardiol Rep 2014; 16: 518. [PubMed][CrossRef]
- 42.
Huynh K, Bernardo BC, McMullen JR et al. Diabetic cardiomyopathy: mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol Ther 2014; 142: 375 - 415. [PubMed][CrossRef]
- 43.
Waterhouse DF, Ismail TF, Prasad SK et al. Imaging focal and interstitial fibrosis with cardiovascular magnetic resonance in athletes with left ventricular hypertrophy: implications for sporting participation. Br J Sports Med 2012; 46 (suppl 1): i69 - 77. [PubMed][CrossRef]
- 44.
Calvier L, Miana M, Reboul P et al. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler Thromb Vasc Biol 2013; 33: 67 - 75. [PubMed][CrossRef]
- 45.
Broberg CS, Burchill LJ. Myocardial factor revisited: The importance of myocardial fibrosis in adults with congenital heart disease. Int J Cardiol 2015; 189: 204 - 10. [PubMed][CrossRef]
Torvald Espeland og kolleger har skrevet en verdifull og interessant oversikt over myokardfibrose, men utelater å nevne et viktig kardiologisk fagfelt ved myokardfibrose, nemlig medfødte hjertefeil hos både barn og voksne. Medfødt hjertefeil forekommer hos ca. 1% av nyfødte. Langtidskomplikasjoner hos undergrupper med medfødte hjertefeil rammer derfor et betydelig antall pasienter, ofte i relativt ung alder.
I flere tiår har man hatt kunnskapen om fibroseutvikling som en bekymringsverdig langtidskomplikasjon tross kirurgisk reparasjon av hjertefeilen tidlig i livet (1). Fibroseutviklingen synes hos disse pasientene ikke kun å være knyttet til hjertefeilens alvorlighetsgrad eller cyanose, men også til genetiske faktorer. Myokardfibrose er en viktig medvirkende årsak til hjertesviktutvikling hos voksne med medfødte hjertefeil (2). Hos pasienter med enkel ventrikkel bidrar tidlig fibroseutvikling sannsynligvis til pumpesvikt i barnealder (3).
MR-undersøkelse med T1-mapping og beregning av ekstracellulært volum kan gi unik informasjon om myokard og grad av diffus fibrose, men er noe mer komplisert å bruke enn angitt i artikkelen. Metoden for T1-mapping er maskinspesifikk og ennå ikke helt standardisert for klinisk bruk. T1-relaksasjonstiden påvirkes av flere faktorer og forskjellige tekniske parametre, og ekstracellulært volum anses som en mer robust parameter. Derfor er det anbefalt å etablere lokale referanseverdier for å oppnå reproduserbare målinger av god kvalitet (4). Økt diffus myokardfibrose er påvist med T1-mapping ved ulike typer hjertefeil som Fallots tetrade, medfødt aortastenose, enkel ventrikkel og transposisjon av de store arterier (5).
Myokardfibrose er en viktig faktor for utvikling av nedsatt myokardfunksjon ved ervervede og medfødte hjertesykdommer. Korrekt anvendt kan MR-teknikker hjelpe med å forstå og kartlegge fibroseprosessen.
Litteratur:
1. Rathod RH, Powell AJ, Geva T. Myocardial Fibrosis in Congenital Heart Disease. Circ J 2016; 80: 1300-7.
2. Broberg CS, Burchill LJ. Myocardial factor revisited: The importance of myocardial fibrosis in adults with congenital heart disease. Int J Cardiol 2015; 189: 204-10.
3. Sugimoto M, Saiki H, Tamai A et al. Ventricular fibrogenesis activity assessed by serum levels of procollagen type III N-terminal amino peptide during the staged Fontan procedure. J Thorac Cardiovasc Surg 2016; 151: 1518-26.
4. Messroghli DR, Moon JC, Ferreira VM et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 2017; 19: 75.
5. Riesenkampff E, Messroghli DR, Redington AN et al. Myocardial T1 mapping in pediatric and congenital heart disease. Circ Cardiovasc Imaging 2015; 8: e002504.
Vi vil gjerne takke for relevant kommentar til vår artikkel om myokardfibrose. Pålagt begrensning på artikkelens størrelse medførte at vi måtte utelate flere viktige tema. Vi valgte derfor å fokusere på klassifisering, patofysiologi og påvisningsmuligheter og omtale noen sentrale sykdomstilstander. I første innsendte versjon var imidlertid også pasienter med medfødt hjertefeil inkludert, og vi er glade for at denne pasientgruppen igjen får oppmerksomhet.
Pasienter med medfødt hjertefeil har ofte alvorlig hjertesykdom som påvirker pasienten gjennom hele livsløpet (1). Möller og de Lange nevner at myokardfibrose medvirker til hjertesviktutvikling hos voksne med medfødt hjertefeil, og at genetiske forhold påvirker fibroseomfang blant pasienter med medfødte hjertefeil. Genetiske forhold spiller trolig også en rolle for hvordan ervervet hjertesykdom fører til utvikling av myokardfibrose, både med tanke på type og mengde fibrose. Individvariasjoner forklares neppe av forskjeller i sykdomsbelastning alene. Fibrose står sentralt i utviklingen av nedsatt hjertefunksjon og disponerer for arytmier ved mange hjertesykdommer.
Flere sykdomstilstander hadde fortjent omtale i artikkelen. Vi vil i tillegg nevne kreftoverlevere som har gjennomgått kardiotoksisk kjemoterapi eller strålebehandling mot brystregionen. Med bedret kreftoverlevelse vil denne pasientgruppen øke, og de vil ha økt risiko for å utvikle fibrose i myokard og hjertet forøvrig. Slike bivirkninger vil ofte påvirke livskvalitet, sykelighet og dødelighet (2).
Videre påpeker Möller og de Lange at ekstracellulært volum anses som en mer robust parameter enn nativ T1-mapping. Dette er vi enige i, og metoden har som nevnt høyt samsvar med histologisk interstitiell fibrose. I tillegg til lokale referanseverdier kreves hematokritmåling for standard beregning av ekstracellulært volum. For å unngå endringer i erytrocyttvolumfraksjon er det ønskelig med kortest mulig tid mellom blodprøvetaking og MR-undersøkelse. Utvikling av medisinsk teknologi har de siste årene muliggjort syntetisk beregning av ekstracellulær volum, med avledning av denne verdien basert på blodets egen T1-verdi (3). Det er ingen tvil om at MR har bidratt til økt forståelse og bedre kartlegging av myokardfibrose.
Litteratur
1. Broberg CS, Burchill LJ. Myocardial factor revisited: The importance of myocardial fibrosis in adults with congenital heart disease. Int J Cardiol 2015; 189: 204-10.
2. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016; 37: 2768-801.
3. Messroghli DR, Moon JC, Ferreira VM, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 2017; 19: 75.