Substance dependence is a disorder that leads to impaired mental, physical and social functioning, and that often shows progressive development and a chronic course (1). Alcohol dependence and substance dependence/abuse are considered to be among the most common causes of poor health and are ranked as the third leading cause of disability-adjusted life years (2).
Proper detoxification is the gateway to further treatment of substance dependence. A detoxification unit has three basic tasks: safe detoxification, identifying and clarifying status of disease, and motivating the patient to engage in further treatment. Prior to the Social Services Act of 1993, detoxification was performed mainly on alcohol-dependent middle-aged men. The age of patients has subsequently decreased, and the problematic use of two or more substances simultaneously (hereafter referred to as «polydrug» use), for example opioids and benzodiazepines, is now seen in up to 80 % of patients in specialised detoxification units (3). The use of combinations of substances complicates detoxification, and requires additional expertise regarding the withdrawal symptoms that occur as the various substances leave the body.
Treatment at a detoxification centre is demanding, in terms of both the social and the pharmacological components. Alcohol, opioids and benzodiazepines are the individual substances that cause the greatest discomfort and most complications in the detoxification process. There are well-established guidelines for detoxification from each of these substances individually (4) – (7). However, guidelines for pharmacological detoxification of patients with polydrug use are lacking. There are no meta-analyses or clinical knowledge summaries available, just a few individual studies (8) – (11).
The Norwegian Directorate of Health’s national guidelines for medical professionals on detoxification from substance abuse and addictive medications recommend inpatient treatment for simultaneous detoxification from multiple substances. However, the Directorate avoids taking a position on the pharmacological treatment regimen to be used. Instead, the new guidelines describe withdrawal treatment for the most common substances with potentially complicated detoxification, for each substance individually (7).
Therapeutic options
There are clinically two main pharmacological regimes for polydrug detoxification. The first uses opioid– and benzodiazepine receptor agonists given in tapering doses. The second regimen uses symptom-reducing medications not involved in the misuse pattern.
The preferred agents for alleviation of withdrawal symptoms are anticonvulsants and alpha-adrenergic agonists such as clonidine and lofexidine (8, 12, 13). The advantages of these medications are that they do not induce tolerance and do not have dependence potential. Benzodiazepines are frequently part of the pattern of substance abuse. Use of benzodiazepines in the context of detoxification may contribute to cravings and may also pose challenges in working with patients to achieve appropriate dosing and discontinuation.
Benzodiazepine- and opioid-free detoxification offers the advantages of avoiding the dependence and abuse potential of these substances, as well as their CNS and respiratory depressant effects. Furthermore, if alcohol is involved, one can begin treatment while the blood alcohol level is falling, and thereby potentially shorten the duration of acute life-threatening withdrawal symptoms. The feasibility and efficacy of this type of treatment for withdrawal symptoms in cases of polydrug use should therefore be examined.
Research questions
In this study we sought to describe the pattern of substance use among polydrug users presenting to a specialist detoxification unit, and to answer the following questions
Did patients with mixed withdrawal symptoms complete detoxification in a unit with standardised symptom management, and to what extent did complications arise during the detoxification? To what degree did procedures deviate from the unit’s standardised pharmacological detoxification protocol?
Material and method
The study was conducted as a non-experimental cohort study without a defined control group. Data for the year 2013 were collected at the Addiction Unit of Sørlandet Hospital in Kristiansand.
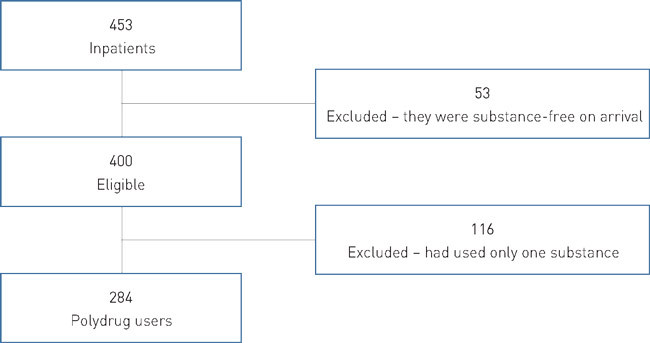
All patients who reported on admission that they had been using two or more substances, with usage confirmed by alcohol breath test and urinary drug screening, were included in the dataset (Fig. 1).
Treatment
Social therapy and medications were used to manage withdrawal symptoms. Social therapy entailed establishing a fixed and predictable structure, building relationships, gathering and clarifying information, motivational work, education and physical activity.
Standardised pharmacological treatment comprised clonidine and valproate in tablet form. Clonidine is a central alpha-2-adrenergic agonist which counteracts opioid withdrawal symptoms associated with noradrenergic hyperactivity (4). The agent offers relief for patients with dominant opioid withdrawal by reducing restlessness, muscle twitching and stress (4). Its efficacy is well documented in patients with mild to moderate symptoms (12). Clonidine may cause adverse effects, including hypotension and insomnia, especially in the first few days. A dosage of 50 – 150 μg three times daily was used until urine toxicology tests were negative for opioids, usually after 6 – 10 days.
Valproate is an antiepileptic that acts on brain GABA-A receptors. It has been used in our unit since 2002 to alleviate withdrawal symptoms and to prevent seizures and delirium tremens in patients with alcohol or benzodiazepine withdrawal. Its efficacy in preventing seizures and alleviating symptoms is similar to that of carbamazepine (13), but carbamazepine interacts with opioids (14) and has more adverse effects (15). Valproate should not be given to pregnant women (16). The dosage used was Orfiril long 600 mg twice daily. To quickly attain an adequate serum concentration, a loading dose of 1200 mg was given in oral solution. Orfiril was administered until benzodiazepines could no longer be detected in the urine.
Additional symptomatic treatment was determined on the basis of individual assessment: alimemazine was used for sleep disturbances; hydroxyzine for anxiety and agitation; paracetamol for somatic pain; ibuprofen for muscular pain; lactulose for constipation, metoclopramide for nausea, and loperamide for diarrhoea. Neuroleptics such as chlorprothixene and levomepromazine were used occasionally for sleep disturbances. Patients also received a daily dose of vitamins, and adequate fluids and nutrition.
The duration of treatment of withdrawal symptoms in this study was set to ten days. This was based on a previous study of polydrug users (8) and on detection times for the individual substances in the urine (17). Clinical experience has shown that the risk of severe withdrawal symptoms is related to drug detection time in the urine.
In this study, treatment was considered completed after ten days in the unit or upon a negative urine toxicology screen. An exception was made for cannabis, which can be detected in the urine for several weeks.
The Regional Ethics Committee (REC) considered the study to be a Quality Assurance Project exempt from the requirement of ethical approval. The study was approved by the Norwegian Centre for Research Data.
Statistics
Descriptive statistics were used to describe the patient cohort. Multiple logistic regression was used to identify the variables associated with treatment discontinuation. Odds ratios (ORs) were presented with 95 % confidence intervals (CIs), and the statistical significance level was set to 5 %. Data were analysed using IBM SPSS version 21.0
Results
Pattern of substance abuse in patients
In total, 284 (71 %) of all eligible patients admitted to the unit for detoxification in 2013 were polydrug users. Demographic data and the three most common usage patterns are shown in Table 1. The average age was 39 years, but with a wide range spanning 18 – 70 years. The average duration of hospitalisation was nine days, and polydrug use was three times more common in men than in women.
Table 1
Characteristics of enrolled patients with polydrug use attending the Addiction Unit, Sørlandet Hospital, Kristiansand in 2013 (n = 284, mean age 39 years, range 18 – 70 years)
| Number |
(%) |
|
| Sex, percentage female |
68 |
(24) |
| Number of substances used |
||
| Two substances |
131 |
(46) |
| Three substances |
91 |
(32) |
| Four or more substances |
62 |
(22) |
| Most common substance combinations |
||
| Alcohol, benzodiazepines |
40 |
(14) |
| Benzodiazepines, cannabis, opiates and amphetamine |
39 |
(14) |
| Benzodiazepines, cannabis and amphetamine |
23 |
(8) |
Each patient used an average of 3.2 different addictive substances (range 2 – 8). A total of 32 different polydrug combinations were seen. Cannabis was present in 57 % of combinations and benzodiazepines in 56 %, followed by opioids in 41 %, alcohol in 32 %, amphetamine/methamphetamine in 28 % and cocaine in 2 %.
Combinations including at least one CNS depressant predominated. Eighty-two patients who used stimulants such as amphetamine, methamphetamine and cocaine combined these with CNS depressants such as benzodiazepines, opioids, alcohol and cannabis.
Treatment completion
In all, 75 % of patients (213 of 284) completed the treatment, whereas 25 % (71 patients) discontinued. The number of discontinuations conservatively included all those who changed their mind at the last minute. Thus eighteen patients dropped out on the first day owing to loss of motivation at the time of admission. Discontinuations were otherwise evenly distributed over the 10-day period. Reasons for discontinuation are shown in Table 2.
Table 2
Of 284 patients, 71 (25 %) discontinued treatment. Reasons for discontinuation
| Reason |
Number |
(%) |
| Lost motivation |
27 |
(38.0) |
| Expelled |
9 |
(12.8) |
| Transferred to somatic ward |
6 |
(8.1) |
| Transferred to psychiatric ward |
4 |
(5.6) |
| Disagreement over medication |
2 |
(2.8) |
| Unknown/other |
23 |
(32.4) |
| Total |
71 |
(100) |
In a regression analysis controlling for sex and age, there was a weak but significant correlation between treatment discontinuation and the number of substances used (OR 1.42; p = 0.042) (Table 3).
Table 3
Odds ratio for treatment discontinuation by sex, age and number of substances used. Multivariate logistic regression analysis. Explained variance = 3 % (Nagelkerke R2)
| OR (95 % CI) |
P-value |
|
| Sex |
0.92 (0.48 – 1.77) |
0.81 |
| Age |
0.99 (0.96 – 1.01) |
0.33 |
| Age |
0.99 (0.96 – 1.01) |
0.33 |
| Number of substances |
1.42 (1.01 – 1.99) |
0.04 |
Complications
The incidence of complications was very low. Delirium tremens occurred in 1.1 % of patients, epileptic seizures in 1.1 % and substance-induced psychosis in 1.4 %. Two patients with known schizophrenia diagnoses who were psychotic upon arrival were excluded from the analysis. Serious somatic complications during hospitalisation (1.8 %) included cardiac problems, infections and poorly controlled diabetes.
Six patients were transferred to a somatic ward and four to a psychiatric ward with complications that were too severe for treatment in the detoxification unit.
Deviations from standardised pharmacological treatment
Standard treatment with valproate and clonidine was used in 254 (89 %) of 284 cases. A total of 23 patients (8 %) received clomethiazole, all of whom had been using alcohol in combination with benzodiazepines prior to admission. Twelve of these patients had used only benzodiazepines and alcohol, while 11 had used a number of other substances in addition, including opioids, cannabis and stimulants.
Severe withdrawal symptoms led to clomethiazole administration in seven cases: three cases of predelirium/delirium, two cases of withdrawal seizures, and two cases of psychosis associated with withdrawal. For the remaining patients, clomethiazole was administered due to severe sleep disturbances and reports of delirium or epileptic seizures in their anamneses.
Standard treatment with clonidine was insufficient for six patients (2 %) with dominant opioid abuse in combination with amphetamines, benzodiazepines and cannabis. These patients received supplementary treatment with buprenorphine for six days. Only one patient with extensive alcohol and benzodiazepine abuse received a benzodiazepine (oxazepam) during hospitalisation, requested by the general practitioner and rehabilitation institute receiving the patient.
Discussion
Pattern of substance abuse
Polydrug use predominated among patients admitted for detoxification. During the study period, 71 % experienced withdrawal symptoms linked to at least two substances. There were 32 different combinations in total. The most common combination was alcohol and benzodiazepines, followed by the combination benzodiazepines, cannabis, opiates and amphetamine.
Compared with Hobbesland’s study ten years ago (3), the prevalence of cannabis and opioid use upon admission to a detoxification unit had risen, the use of benzodiazepines had remained largely unchanged, while the use of alcohol and stimulants had decreased.
Treatment completion
The current study found that three quarters of polydrug users completed a brief benzodiazepine-free detoxification with the aid of standard symptom-reducing medications not involved in the substance abuse pattern.
Completion of detoxification is a first and essential step in treatment and rehabilitation. The completion rates reported in the literature vary between treatment regimes and populations (8, 9) – (11), but are generally low. For example, Hobbesland reported a completion rate of 55 % (3).
Discontinuation is unfortunate as it often entails disappointment and disillusionment for the patient and her/his family. It is also frustrating for the therapists, and opioid users have a markedly elevated risk of overdose in the period following discharge (18). The high completion rate in our study is thus encouraging.
But even with a completion rate of 75 %, there is still room for improvement. The study showed that the greater the number of substances used, the greater the risk of discontinuation. Cravings and ambivalence may lead to discontinuation, as may overly severe withdrawal symptoms. In this study, however, only two patients reported that they discontinued treatment because of dissatisfaction with the medication used. In addition, only a small number of patients were discharged against their will because of threatening or aggressive behaviour or the use of substances that had been smuggled in.
Complications
The treatment was free of complications in 95 % of cases. Complications like delirium tremens, epileptic seizures and psychosis (3.6 %) were rare. Severe somatic complications that were not necessarily due to withdrawal treatment, but might instead be the result of prolonged substance abuse, accounted for less than 2 %. Ten patients were transferred to somatic or psychiatric wards.
By comparison, Hobbesland (3) reported a complication rate that was twice as high (7.0 %) in his study from six detoxification centres in Southern Norway, where treatment was not standardised. Seizures were described in 1.9 % of patients, delirium tremens in 1.9 % and substance-induced hallucinations/psychosis in 3.2 %. In addition, more patients in that study were transferred to somatic and psychiatric hospital wards (5.3 % versus 3.5 %).
Departures from standard treatment/supplementary pharmacotherapy
Eighty-nine percent of patients followed the standardised treatment regimen. Benzodiazepines were used in only a single case, while 8 % of patients received supplementary treatment with clomethiazole. This was mainly in cases of polydrug use involving alcohol or benzodiazepines. Considering that alcohol was used by one third of polydrug users, and benzodiazepines by more than half, this number can be regarded as low. The advantages of not using benzodiazepines (as previously discussed) include a shorter duration of inpatient treatment. Only 2 % of patients received supplementary treatment with buprenorphine: it was given to six patients who were using opioids in combinations for which treatment with clonidine was insufficient.
It would be useful to have comparable figures for the detoxification of polydrug users using other regimes, such as tapering with decreasing doses of an opioid plus a benzodiazepine, but searches in PubMed and PsychInfo for such comparable studies revealed a gap in the existing knowledge base. On the basis of a few small experimental studies (8, 9) – (11), one would expect dropout rates during polydrug withdrawal involving opioids to be higher with the use of clonidine than with tapering. Ling et al. (11) found, for example, that 77 % completed 13 days of detoxification with buprenorphine/naltrexone, compared with 22 % with clonidine in an opioid-dominated polydrug population.
Since our study showed markedly higher completion rates, the explanation may lie in factors other than the purely pharmacological, most notably in the social therapy. The Swedish Board of Health and Welfare’s guidelines for addiction treatment (19) emphasise the quality of the social environment and the approach and availability of personnel as key elements of withdrawal management. During the current study the unit had stable personnel with extensive experience. Predictable and well-established routines in combination with effective medication may have contributed significantly to the high completion rate, low rate of complications, and limited deviations from the standard treatment regime.
Methodological strengths and weaknesses
There has been little discussion of standardised treatment regimens for detoxification of polydrug users, and neither Norwegian nor international guidelines exist. Patients were enrolled over an entire calendar year, as severe withdrawal complications are relatively rare and a broad dataset increases the study’s level of accuracy.
The study was conducted as a non-experimental cohort study without a defined control group. A randomised, controlled study is recommended to compare the treatment regimen described here with a benzodiazepine-opioid regimen in order to quantify treatment efficacy.
Conclusion
The results of the current study show a higher completion rate and a lower incidence of severe withdrawal complications than previous comparable studies.
The treatment regimen examined in this study appears to be safe, robust and effective, and deserves to be regarded as a benzodiazepine-free first-line standardised withdrawal treatment in cases of polydrug use.
MAIN POINTS
Patients admitted to the Detoxification Unit of Sørlandet Hospital in 2013 were predominantly polydrug users
The current study observed few complications and high completion rates for benzodiazepine-free detoxification with clonidine and valproate
The study revealed a weak but statistically significant correlation between the number of substances used and the risk of treatment discontinuation
- 2.
WHO. Global health risks – mortality and burden of disease attributable to selected major risks.: www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf (12.3.2016).
- 3.
Hobbesland Å. Undersøkelse av avgiftningsbehandling ved seks avgiftningsavdelinger i helseregion sør. Skien: Borgestadklinikken, 2006.
- 4.
Shaygani S, Waal H. Behandling av opioid abstinens. Tidsskr Nor Legeforen 2009; 129: 114 – 5. [PubMed]
- 5.
Helland A, Skjøtskift S. Medikamentell behandling av alkoholabstinens. Tidsskr Nor Laegeforen 2008; 128: 1182 – 4. [PubMed]
- 7.
Nasjonal faglig retningslinje for avrusning fra rusmidler og vanedannende legemidler. https://helsedirektoratet.no/retningslinjer/avrusning-fra-rusmidler-og-vanedannende-legemidler (12.3.2016).
- 12.
Gowing L, Farrell MF, Ali R et al. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev 2014; 3: CD002024. [PubMed]
- 13.
Minozzi S, Amato L, Vecchi S et al. Anticonvulsants for alcohol withdrawal. Cochrane Database Syst Rev 2010; nr. 3: CD005064. [PubMed]
- 14.
Saxon AJ, Whittaker S, Hawker CS. Valproic acid, unlike other anticonvulsants, has no effect on methadone metabolism: two cases. J Clin Psychiatry 1989; 50: 228 – 9. [PubMed]
- 16.
Nakken KO, Taubøll E. Valproat bør unngås hos gravide. Tidsskr Nor Legeforen 2014; 134: 144 – 5. [PubMed]
- 17.
Rusmiddelanalyser. Prøvetaking, analyser og fortolkning. www.oslo-universitetssykehus.no/omoss_/avdelinger_/farmakologi_/Documents/Rusmiddelheftet%20revidert%20101213%20%283%29.pdf. (12.3.2016)
- 19.
Nationella riktlinjer för missbruks- och beroendevård. www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/19770/2015 – 4 – 2.pdf. (14.3.2016)
Ånund Hobbesland har gjort oss oppmerksom på at vår studie (1) og hans studie fra 2006 (2) ikke er direkte sammenlignbare når det gjelder gjennomføringsgrad. Dette henger sammen med at definisjonen av endepunktet for fullført detoksifisering var noe forskjellig i de to studiene. Vår studie hadde to kriterier: gjennomført ti døgns behandling og ingen påvisbare rusmidler i urin. Hobbesland sin studie hadde fire kriterier: gjennomført etter anbefaling fra personale, ingen påvisbare rusmidler i urin, fravær av «svært mye» abstinensplager og fravær av høyt nivå av psykiske plager. Hobbesland beskrev frafall i hele populasjonen (både detoksifisering av brukere av ett enkelt rusmiddel og blandingsmisbrukere), mens vi konsentrerte oss om blandingsmisbrukerne. Hobbesland fant, med sine noe strengere kriterier, et frafall i gjennomføringsgrad på 45% (2), mens vi i vår studie bare hadde 25% (1).
Vi vil derfor presisere at forskjellen mellom studiene kanskje derfor ikke er så stor. Gjennomføringsgraden for avvenning av blandingsmisbrukere er imidlertid generelt lav. Litteratursøk i Pubmed og PsychInfo ga oss 22-77% (2-6). Studien vår viste en gjennomføringsgrad på 75%.
Et for oss viktigere poeng er at standardisert symptomatisk behandling med klonidin og valproat er trygg og gjennomførbar med lite komplikasjoner. Dette er vårt hovedbudskap, all den stund Helsedirektoratets retningslinjer for avrusning fra 2016 (7) unngår å ta stilling og behandlingsregimene rundt om i landet er i endring.
Forskningsbasert kunnskap om hvilken behandling som er mest velegnet ved avvenning fra blandingsmisbruk, mangler. Retningslinjer er ønskelig ettersom pasienter med blandingsmisbruk og blandingsavhengighet dominerer i en avrusningspost i dag. Derfor har vi lenge etterlyst randomiserte kontrollerte studier med standardiserte behandlingspakker. Et kvalitetsregister ville også kunne gi nyttig kunnskap og danne grunnlag for bedre retningslinjer.
Litteratur
1. Dunsæd F, Kristensen Ø, Vederhus J-K et al. Standardisert avrusning ved blandingsmisbruk. Tidsskr Nor Legeforen nr. 19, 2016; 136: 1639 – 42
2. Hobbesland Å. Undersøkelse av avgiftningsbehandling ved seks avgiftningsavdelinger i helseregion sør. Skien: Borgestadklinikken, 2006.
3. Kristensen O, Lolandsmo T, Isaksen A, et al. Treatment of polydrug-using opiate dependents during withdrawal: towards a standardisation of treatment. BMC psychiatry 2006; 6: 54.
4. Paetzold W, Eronat V, Seifert J, et al. Detoxification of poly-substance abusers with buprenorphine. Effects on affect, anxiety, and withdrawal symptoms. Der Nervenarzt 2000; 71: 722-9.
5. Seifert J, Metzner C, Paetzold W, et al. Detoxification of opiate addicts with multiple drug abuse: a comparison of buprenorphine vs. methadone. Pharmacopsychiatry 2002; 35: 159-64.
6. Ling W, Amass L, Shoptaw S, et al. A multi-center randomized trial of buprenorphine-naloxone versus clonidine for opioid detoxification: findings from the National Institute on Drug Abuse Clinical Trials Network. Addiction 2005; 100: 1090-100
7. Nasjonal faglig retningslinje for avrusning fra rusmidler og vanedannende legemidler. https://helsedirektoratet.no/retningslinjer/avrusning-fra-rusmidler-og-vanedannendelegemidler (01.11.2016)