Mode of delivery after induction of labour in pregnant women with pre-pregnancy overweight and obesity
Main findings
Of the 807 women whose labour was induced at the University Hospital of North Norway, Tromsø in the period 2016–18, 203 (25 %) had pre-pregnancy obesity.
The proportion of pregnant women whose labour was induced and who had a spontaneous vaginal delivery with a pre-pregnancy BMI <25 kg/m2, overweight and obesity were 306 (76 %), 149 (74 %) and 140 (69 %), respectively. The corresponding figures for caesarean section were 60 (15 %), 40 (20 %) and 44 (22 %).
Women with pre-pregnancy obesity whose labour was induced had a higher risk of caesarean section than those without overweight or obesity.
Overweight and obesity are major public health challenges (1). The prevalence of pre-pregnancy obesity in pregnant women in Norway increased from 12 % in 2007 to 13 % in 2018 and 16 % in 2023 (2). Overweight and obesity are associated with complications in pregnancy and childbirth (3, 4), as well as an increased risk of induced labour (5–7).
The proportion of induced labour in Norway increased from 9 % in 2000 to 24 % in 2018 and to 30 % in 2023 (2, 8). The World Health Organization recommends that induction of labour should be performed only when there is a clear medical indication for it, and the expected benefits for mother or child outweigh the risk of continuing the pregnancy (8, 9). Methods used to induce labour include insertion of a balloon catheter through the cervix, drugs administered orally, vaginally or intravenously, and amniotomy (8, 10).
Caesarean section may be indicated for maternal reasons, fetal reasons or both. Women without a previous vaginal delivery have an increased risk of caesarean section (11). The rate of caesarean sections in Norway was 16 % in 2005, 2018 and 2023 (2). Among 1818 nulliparous women whose labour was induced at 21 Norwegian birth units in 2018, caesarean section rates ranged from 9 % to 46 % (11). Another Norwegian study, based on data from 8821 pregnant women in 2011–12, demonstrated that obesity doubled the risk of caesarean section in both nulliparous and multiparous pregnant women (12). There are no published figures in Norway for the association between pre-pregnancy body mass index (BMI) and mode of delivery after induction of labour.
Since rates of both pre-pregnancy overweight and obesity as well as induction of labour are increasing, we wanted to study whether there was a relationship between pre-pregnancy BMI and mode of delivery in pregnant women whose labour was induced.
Material and method
The study was a quality assurance project and included all pregnant women whose labour was induced at the Department of Obstetrics and Gynaecology, University Hospital of North Norway, Tromsø in the period from 1 January 2016 to 31 December 2018. The women were identified in the Norwegian DIPS medical records system based on the following procedure codes: MAC10 – Induction of labour with balloon catheter, MAC96 – Other surgical induction of labour, MAGM05 – Induction of labour with oral drug, MAGM10 – Induction of labour with intravenous drug and MAGM16 – Induction of labour with locally administered drug.
The inclusion criteria were induced labour, singleton pregnancy, fetus in cephalic presentation and a liveborn child after 24 + 0 weeks of gestation. The study was approved by the data protection officer at the University Hospital of North Norway.
We recorded the following details from the medical records: indication for induction of labour and method used at each stage, indication for caesarean section, maternal age, height, parity, country of birth, development of pre-eclampsia and gestational diabetes, previous caesarean section, infant birth weight, macrosomia (birth weight ≥ 4500 grams), gestational age, five-minute Apgar score and transfer to the neonatal unit.
BMI was based on self-reported pre-pregnancy height and weight or measurements in the first trimester recorded on the antenatal health card. The study participants were divided into three categories based on pre-pregnancy BMI: BMI < 25 kg/m2 (reference group), overweight (BMI 25.0–29.9 kg/m2) and obesity (BMI ≥ 30 kg/m2).
The mode of delivery was classified as spontaneous vaginal delivery, caesarean section and operative vaginal delivery (use of vacuum or forceps).
A composite variable for parity and previous mode of delivery grouped the pregnant women as nulliparous, multiparous with previous vaginal delivery (reference group) and multiparous without previous vaginal delivery (previous caesarean delivery only and therefore considered to be 'nulliparous for vaginal delivery').
The characteristics of the population are presented with mean values and standard deviations for continuous variables, and number and proportion for categorical variables. The p-value for trend in the proportion with spontaneous vaginal delivery and caesarean section was estimated using linear regression analyses, with the three categories of BMI as independent variables. The odds ratio (OR) with 95 % confidence interval (CI) for caesarean section was estimated using logistic regression analyses for all participants, and separately for nulliparous and multiparous pregnant women with or without previous vaginal delivery. The OR (95 % CI) for operative vaginal delivery was estimated for all participants with vaginal delivery. The analyses were performed both with and without adjustment for maternal age, height, prolonged rupture of membranes and the composite variable for parity and previous mode of delivery.
We also studied whether the most common indications for induction of labour were unevenly distributed between the BMI categories and whether they affected the association between overweight/obesity and caesarean section. We performed subanalyses for each of the three groups based on the composite variable. The analyses were performed in SAS version 9.4, with p <0.05 as the significance level.
Results
In total, 913 (22.4 %) of the 4082 pregnant women at the University Hospital of North Norway, Tromsø had labour induced in the period 2016–18, of whom 807 (88.4 %) met the inclusion criteria and were included in the study. The other 106 women were not included due to twin pregnancies (n = 33), intrauterine fetal death (n = 9), breech position (n = 9), or due to a lack of data about pre-pregnancy BMI or labour induction method (n = 55).
Pregnant women with a pre-pregnancy BMI < 25 kg/m2 (the reference group) accounted for 402 (49.8 %, including 23 (2.9 %) with a BMI < 18.5 kg/m2), while 202 (25.0 %) were overweight, and 203 (25.2 %) were obese (Table 1). The most common indications for induction of labour were prolonged rupture of membranes (n = 187, 23.2 %), overdue pregnancy (n = 148, 18.3 %) and hypertensive pregnancy complications (n = 126, 15.6 %). The most common first-line method of inducing labour was balloon catheter (n = 534, 66.2 %), with the second most common being misoprostol (n = 217, 27.0 %).
Table 1
Maternal characteristics, mode of delivery and neonatal outcome categorised by BMI for 807 pregnant women whose labour was induced at the University Hospital of North Norway, Tromsø in the period 2016–18. Figures are mean ± standard deviation or n (%).
| Reference group BMI < 25.0 kg/m2 | Overweight | Obesity | p for trend1 | ||
|---|---|---|---|---|---|
| Number | 402 | 202 | 203 | ||
| Pre-pregnancy body mass index (BMI, kg/m2) | 21.8 ± 2.0 | 27.0 ± 1.5 | 35.7 ± 4.6 | <0.001 | |
| Height (cm) | 165 ± 6.4 | 166 ± 6.4 | 166 ± 6.5 | 0.05 | |
| Age (years) | 30.2 ± 5.8 | 30.9 ± 5.3 | 30.8 ± 5.5 | 0.12 | |
| Parity and previous mode of delivery | |||||
| Nulliparous | 216 (53.7) | 83 (41.1) | 94 (46.3) | 0.03 | |
| Multiparous | |||||
| With previous vaginal delivery | 169 (42.0) | 99 (49.0) | 91 (44.8) | 0.37 | |
| Without previous vaginal delivery | 17 (4.2) | 20 (9.9) | 18 (8.9) | 0.01 | |
| Non-European country of birth | 43 (10.8) | 30 (15.0) | 16 (8.0) | 0.51 | |
| Gestational diabetes | 15 (3.8) | 17 (8.5) | 35 (17.6) | <0.001 | |
| Pre-eclampsia | 52 (13.0) | 23 (11.4) | 40 (19.8) | 0.05 | |
| Premature birth | 28 (7.0) | 10 (5.0) | 6 (3.0) | 0.04 | |
| Gestational age (weeks) | 39.5 ± 2.0 | 39.7 ± 1.9 | 39.7 ± 1.6 | 0.17 | |
| Indication for induction | |||||
| Prolonged rupture of membranes | 106 (26.4) | 45 (22.3) | 36 (17.7) | 0.02 | |
| Overdue pregnancy | 82 (20.4) | 43 (21.3) | 23 (11.3) | 0.01 | |
| Hypertensive pregnancy complications | 53 (13.2) | 25 (12.4) | 48 (23.7) | 0.002 | |
| Mode of delivery | |||||
| Spontaneous vaginal delivery | 306 (76.1) | 149 (73.8) | 140 (69.0) | 0.06 | |
| Caesarean section | 60 (14.9) | 40 (19.8) | 44 (21.7) | 0.03 | |
| Operative vaginal delivery | 36 (9.0) | 13 (6.4) | 19 (9.4) | 0.97 | |
| Neonatal outcome | |||||
| Birth weight (grams) | 3435 ± 613 | 3592 ± 560 | 3692 ± 547 | <0.001 | |
| Macrosomia (≥ 4500 grams) | 18 (4.5) | 9 (4.5) | 19 (9.4) | 0.02 | |
| Apgar score < 7 at 5 minutes | 8 (2.0) | 5 (2.5) | 4 (2.0) | 0.95 | |
| Admission to neonatal unit | 20 (5.1) | 8 (4.0) | 9 (4.5) | 0.70 | |
1p for trend estimated with linear regression
The cohort had a mean pre-pregnancy BMI of 26.6 kg/m2 (range 13.5–57.1), age of 30.5 years (range 14–48) and gestational age of 40 weeks (range 32–42, with 44 (5.5 %) having a gestational age < 37 weeks). In total, 393 (48.7 %) were nulliparous, 595 (73.7 %) had a spontaneous vaginal delivery, 144 (17.8 %) had a caesarean section and 68 (8.4 %) had an operative vaginal delivery. As BMI increased, a marginally lower proportion of women had a spontaneous vaginal delivery (p for trend 0.06) and a higher proportion had a caesarean section (p for trend 0.03), but there was no significant trend for operative vaginal delivery (p for trend 0.97) (Table 1).
In total, 144 (18 %) had a caesarean section. The likelihood of caesarean section for pregnant women with obesity was OR 1.6 (1.0–2.4) compared with the reference group (Table 2). After adjusting for age, height, prolonged rupture of membranes, parity and previous mode of delivery, OR for caesarean section was no longer statistically significant: 1.5 (0.9–2.4). We found no difference in the proportion of pregnant women with overweight who had a caesarean section compared with the reference group.
Table 2
Risk of caesarean section and operative vaginal delivery in 807 pregnant women whose labour was induced at the University Hospital of North Norway, Tromsø in the period 2016–18. Risk is stated as unadjusted and adjusted odds ratio (OR) with 95 % confidence interval (CI).
| Caesarean section – all | Unadjusted OR (95 % CI)1 | Adjusted OR (95 % CI) | |
|---|---|---|---|
| BMI < 25.0 (kg/m2) | Reference group | Reference group | |
| Overweight: BMI 25.0–29.9 (kg/m2) | 1.4 (0.9–2.2) | 1.3 (0.8–2.1)2 | |
| Obesity: BMI ≥ 30.0 (kg/m2) | 1.6 (1.0–2.4) | 1.5 (0.9–2.4)2 | |
| Age (per 5 years higher age) | 1.3 (1.1–1.6) | ||
| Height (per 5 cm lower height) | 1.3 (1.1–1.5) | ||
| Prolonged rupture of membranes | 0.4 (0.3–0.7) | ||
| Parity and previous mode of delivery | |||
| Nulliparous | 3.6 (2.2–5.8) | ||
| Multiparous with previous vaginal delivery | Reference group | ||
| Multiparous without previous vaginal delivery | 18.1 (9.1–36.1) | ||
| Caesarean section – nulliparous | Unadjusted OR (95 % CI) | Adjusted OR (95 % CI) | |
| BMI < 25.0 (kg/m2) | Reference group | Reference group | |
| Overweight: BMI 25.0–29.9 (kg/m2) | 1.4 (0.8–2.6) | 1.5 (0.8–2.8)3 | |
| Obesity: BMI ≥ 30.0 (kg/m2) | 1.4 (0.7–2.5) | 1.5 (0.8–2.8)3 | |
| Age (per 5 years higher age) | 1.3 (1.0–1.6) | ||
| Height (per 5 cm lower height) | 1.4 (1.1–1.7) | ||
| Prolonged rupture of membranes | 0.3 (0.2–0.6) | ||
| Caesarean section – multiparous | Unadjusted OR (95 % CI) | Adjusted OR (95 % CI) | |
| BMI < 25.0 (kg/m2) | Reference group | Reference group | |
| Overweight: BMI 25.0–29.9 (kg/m2) | 1.6 (0.8–3.1) | 1.2 (0.6–2.5)4 | |
| Obesity: BMI ≥ 30.0 (kg/m2) | 2.0 (1.1–3.8) | 1.6 (0.7–3.3)4 | |
| Age (per 5 years higher age) | 1.4 (1.1–1.9) | ||
| Height (per 5 cm lower height) | 1.1 (0.9–1.4) | ||
| Prolonged rupture of membranes | 0.7 (0.3–1.6) | ||
| No previous vaginal delivery | 18.1 (9.0–36.3) | ||
| Operative vaginal delivery – all | Unadjusted OR (95 % CI) | Adjusted OR (95 % CI) | |
| BMI < 25.0 (kg/m2) | Reference group | Reference group | |
| Overweight: BMI 25.0–29.9 (kg/m2) | 0.7 (0.4–1.4) | 0.9 (0.4–1.7)2 | |
| Obesity: BMI ≥ 30.0 (kg/m2) | 1.2 (0.6–2.1) | 1.3 (0.7–2.3)2 | |
| Age (per 5 years higher age) | 1.6 (1.2–2.1) | ||
| Height (per 5 cm lower height) | 1.2 (1.0–1.5) | ||
| Prolonged rupture of membranes (yes/no) | 0.6 (0.3–1.1) | ||
| Parity and previous mode of delivery: | |||
| Nulliparous | 6.3 (3.2–12.2) | ||
| Multiparous with previous vaginal delivery | Reference group | ||
| Multiparous without previous vaginal delivery | 3.8 (1.0–14.6) | ||
1Risk estimated with logistic regression
2Adjusted for age, height, prolonged rupture of membranes, parity and previous mode of delivery.
3Adjusted for age, height and prolonged rupture of membranes.
4Adjusted for age, height, prolonged rupture of membranes and multiparous women without previous vaginal delivery.
The most common indications for caesarean section were imminent foetal asphyxia (n = 64, 44.4 %), slow progression (n = 38, 26.4 %) and hypertensive pregnancy complications (n = 10, 6.9 %). As BMI increased, there was a significantly lower proportion of women with prolonged rupture of membranes (p=0.02), a lower proportion with overdue pregnancy (p = 0.01) and a higher proportion with hypertensive pregnancy complications (p = 0.002). Prolonged rupture of membranes was the only indication for induction that was associated with caesarean section, and it was therefore included in the models for estimating the risk of caesarean section.
Compared with pre-pregnancy obesity, there was a stronger association between being nulliparous or multiparous without previous vaginal delivery and caesarean section (Table 2). In separate analyses of nulliparous and multiparous pregnant women, we found an increased OR for caesarean section in patients with overweight and obesity, but this was not statistically significant. Pre-pregnancy obesity was not associated with operative vaginal delivery in the women who had a vaginal delivery.
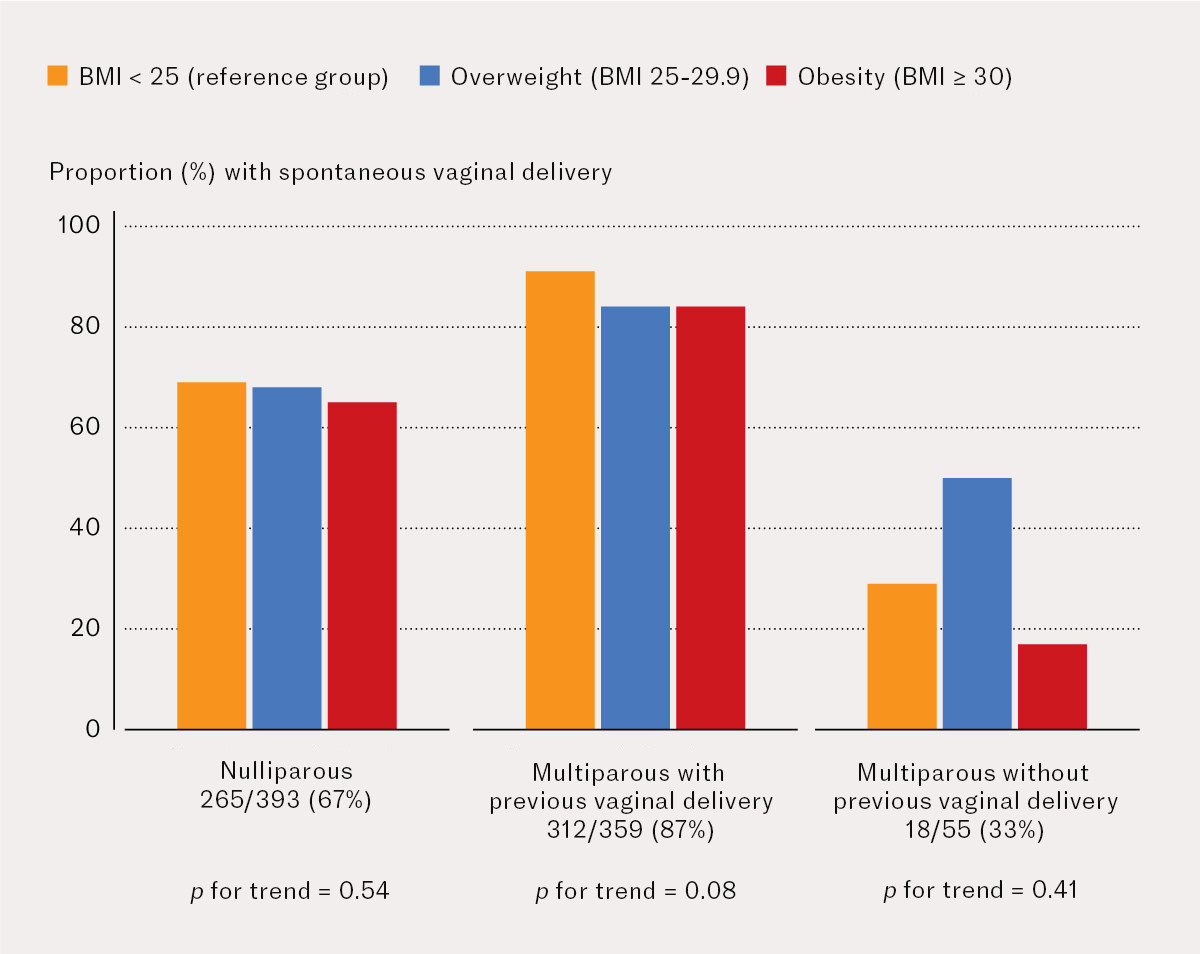
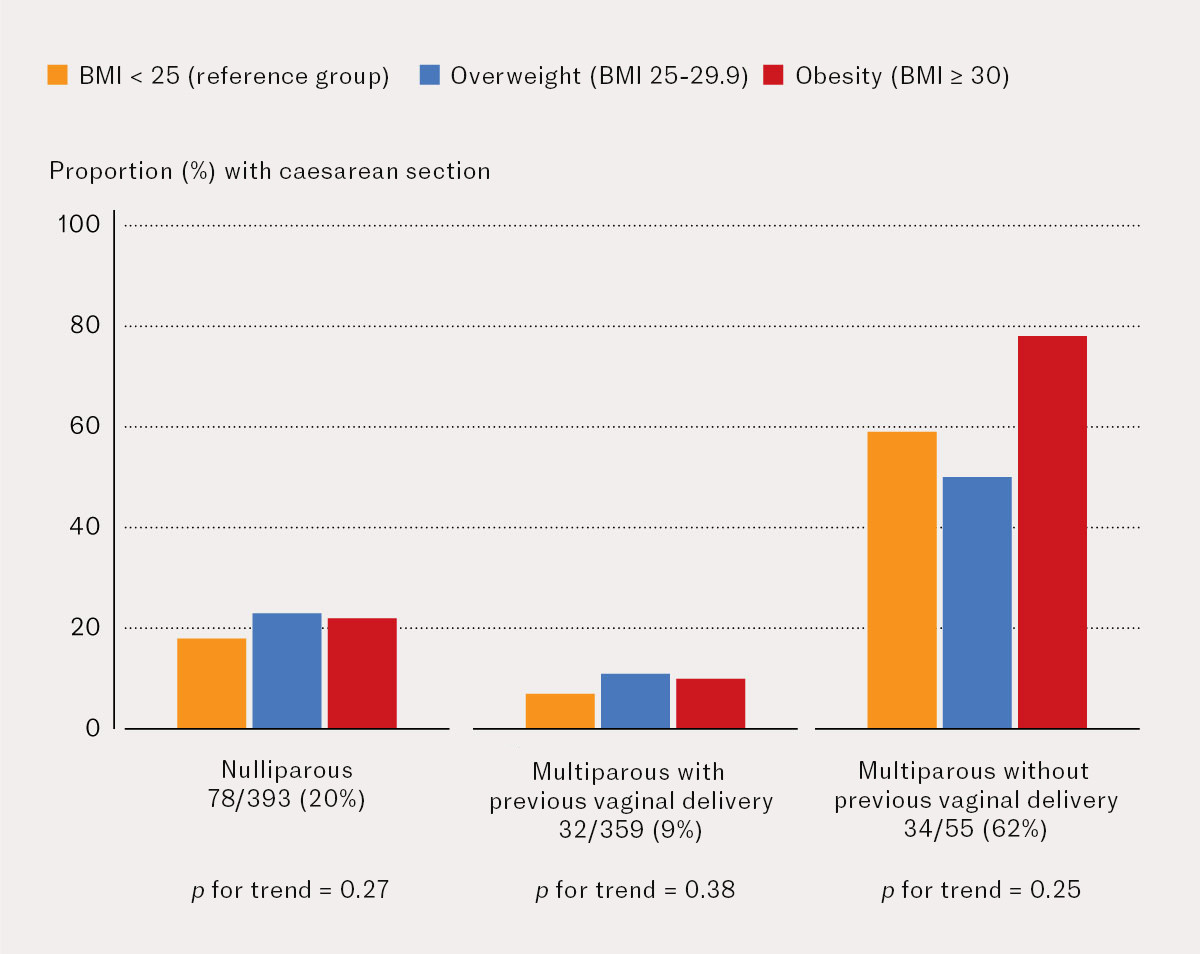
Out of 393 nulliparous pregnant women whose labour was induced, 265 (67.4 %) had a spontaneous vaginal delivery (Figure 1), and 78 (19.9 %) had a caesarean section (Figure 2). Out of 359 multiparous pregnant women with previous vaginal delivery, 312 (86.9 %) had a spontaneous vaginal delivery and 32 (8.9 %) had a caesarean section. Out of 55 multiparous pregnant women without previous vaginal delivery, 18 (32.7 %) had a spontaneous vaginal delivery and 34 (61.8 %) had a repeat caesarean section. There was no statistically significant trend for the proportion with vaginal delivery or caesarean section as BMI increased in any of these subanalyses.
Discussion
In the entire cohort of pregnant women whose labour was induced, obesity was associated with a higher proportion of caesarean sections than BMI < 25 kg/m2 (reference group): 22 % versus 15 %. There was a significant association between obesity and risk of caesarean section in unadjusted analyses, but this association was no longer statistically significant after adjusting for parity and previous mode of delivery. The rationale for this adjustment is that pregnant women without previous vaginal delivery have an increased risk of caesarean section. Being nulliparous or multiparous without a previous vaginal delivery was more strongly associated with a risk of caesarean section than obesity. Seven out of ten pregnant women with obesity whose labour was induced had a spontaneous vaginal delivery, while six out of ten multiparous women without previous vaginal delivery had a repeat caesarean section after induction of labour.
Pregnant women with obesity whose labour was induced had a higher risk of caesarean section in unadjusted analyses. However, our adjusted analyses showed that the association between obesity and risk of caesarean section was no longer statistically significant, as other researchers have previously reported (13, 14). Since the change in the estimate of effect was small (OR 1.6–1.5), we cannot rule out pre-pregnancy BMI having an impact or the increased risk being partly explained by one or more confounding variables. Other research has shown that the risk of emergency caesarean section was doubled in pregnant women with obesity compared with those of normal weight, irrespective of whether their labour had been induced or they had previously undergone a caesarean section (12). Our findings showed that the risk of caesarean section after induction of labour was associated with being nulliparous or multiparous without previous vaginal delivery, irrespective of pre-pregnancy BMI (13, 15). It is generally important to avoid caesarean section in nulliparous pregnant women, and our findings support this. Several earlier studies excluded pregnant women with a previous caesarean section, and it has not therefore been possible to study the effect of previous mode of delivery on the association between obesity and risk of caesarean section in induced labour (13, 16).
Pregnant women with overweight and obesity have longer pregnancies and longer labour, in particular a longer latent phase until 6 cm cervical dilation (16–19). Awareness of this is important in clinical practice to avoid unnecessary interventions.
Prolonged labour in women with obesity can be due to endocrinological and inflammatory changes (18). An increased percentage of body fat and higher volume of distribution can reduce the efficacy of drugs used to induce labour. It has been shown that higher doses are required for successful induction of labour (19). Nevertheless, there is no established practice regarding dose adjustments for obesity when inducing labour (8).
The most common indications for induction of labour (prolonged rupture of membranes, overdue pregnancy and hypertensive pregnancy complications), as well as the methods that were used for induction of labour at the University Hospital of North Norway, Tromsø, were the same as those in a national registration study at 21 Norwegian hospitals in 2018 (8, 11). All three of these main indications for induction of labour were unevenly distributed between the BMI groups, but only prolonged rupture of membranes was associated with caesarean section. The association between obesity and caesarean section was only marginally affected after adjusting for prolonged rupture of membranes when we tested for confounding by the indication for induction of labour. High BMI alone is not an indication for induction of labour, and the management of pregnant women with a high BMI is therefore no different in the absence of the three main indications mentioned above (8).
A lack of association for obesity and overweight in the subanalyses is likely due to low statistical power. The data collection was based on a student assignment in 2020, and this limited the scope of the study. Future studies would be strengthened by using national figures. However, our data were obtained from medical records, and this probably resulted in improved recording of BMI compared with the Norwegian Medical Birth Registry, which was missing 9 % of BMI entries in the study period (2). More recent figures from the Norwegian Medical Birth Registry also provide evidence that our data from 2016–18 are still representative: in 2018 and 2023, the proportion of caesarean deliveries was 16.0 % and 16.2 % respectively nationwide in Norway, and 19.9 % and 19.3 % respectively at the University Hospital of North Norway, Tromsø (2).
We did not study weight over the course of the pregnancy, only pre-pregnancy BMI. However, overweight and obesity in pregnant women have been reported to be associated with caesarean section (13, 17). In our analyses, we chose to adjust for height, even though it is part of the BMI calculation, because lower maternal height is associated with an increased risk of caesarean section (20).
From 2018 to 2023, data from the Norwegian Medical Birth Registry showed a 6 % increase in induced labour both nationally and in Tromsø, as well as a 3–5 % increase in obesity, a one-year increase in the average maternal age, and a 1–2 % increase in nulliparous pregnancies (2). The increase in induced labour is multifactorial, with possible contributing factors being revised definitions of gestational diabetes (2017) and pre-eclampsia (2020) (8). Changes in the definitions have resulted in more women being diagnosed and thus an increased chance of being induced. Other relevant factors include changes in protocols for evaluating overdue pregnancies and an increased awareness of the benefits of inducing labour. It is not known to what extent these changes would have affected the results if the study had a more recent and/or larger data set.
Pre-pregnancy BMI recorded on the antenatal health card is based on self-reported pre-pregnancy height and weight, which is cost-effective and practical but entails an increased risk of reporting bias (21). Nevertheless, earlier studies have shown that self-reported data from the health card is an acceptable and reliable reference for weight (22). Data on BMI or method of induction of labour were missing from 6 % of pregnant women's antenatal health cards, and these women were excluded from the analyses. We consider the proportion of excluded women to be acceptable, but it may have led to underestimation of the risk of complications associated with overweight and obesity. BMI is a useful tool for estimating weight status in a population, but it does not distinguish between muscle mass and fat mass, which can lead to inaccurate assessments of an individual's weight status in women with high muscle mass. We did not have a control group with spontaneous onset of labour, and it was not therefore possible to study differences in mode of delivery between women whose labour was induced and those whose labour started spontaneously.
Conclusion
Pregnant women with obesity had a higher proportion of caesarean sections than pregnant women without overweight or obesity. This risk of caesarean section following induction of labour was predominantly associated with nulliparous or multiparous women without previous vaginal delivery. A clinical implication of our findings is that particular attention should perhaps be paid to women with a previous caesarean delivery when inducing labour, irrespective of their pre-pregnancy BMI.
The article has been peer-reviewed.
- 1.
Midthjell K, Lee CM, Langhammer A et al. Trends in overweight and obesity over 22 years in a large adult population: the HUNT Study, Norway. Clin Obes 2013; 3: 12–20. [PubMed][CrossRef]
- 2.
Folkehelseinstituttet. Medisinsk fødselsregister. https://statistikkbank.fhi.no/mfr/ Accessed 24.2.2025.
- 3.
Fitzsimons KJ, Modder J, Greer IA. Obesity in pregnancy: risks and management. Obstet Med 2009; 2: 52–62. [PubMed][CrossRef]
- 4.
Ovesen P, Rasmussen S, Kesmodel U. Effect of prepregnancy maternal overweight and obesity on pregnancy outcome. Obstet Gynecol 2011; 118: 305–12. [PubMed]
- 5.
Sebire NJ, Jolly M, Harris JP et al. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord 2001; 25: 1175–82. [PubMed]
- 6.
Usha Kiran TS, Hemmadi S, Bethel J et al. Outcome of pregnancy in a woman with an increased body mass index. BJOG 2005; 112: 768–72. [PubMed][CrossRef]
- 7.
El-Chaar D, Finkelstein SA, Tu X et al. The impact of increasing obesity class on obstetrical outcomes. J Obstet Gynaecol Can 2013; 35: 224–33. [PubMed][CrossRef]
- 8.
Oppegaard KS, Dogl M, Sun C et al. Induksjon/igangsettelse av fødsel - Modning av cervix/livmorhalsen før fødsel. Oslo: Norsk gynekologisk forening, 2022. https://www.legeforeningen.no/contentassets/301c9e399250472cabb9009761e673f6/induksjon_igangsettelse-av-fodsel-modning-av-cervix_livmorhalsen-for-fodsel_rev0422.pdf Accessed 23.2.2025.
- 9.
World Health Organization. WHO recommendations for induction of labour. https://apps.who.int/iris/bitstream/handle/10665/44531/9789241501156_eng.pdf?sequence=1 Accessed 6.5.2024.
- 10.
Oppegaard KS, Heimstad R, Lippert T et al. 33. Cervixmodning/induksjon av fødsel. Veileder i fødselshjelp (2014). https://www.legeforeningen.no/foreningsledd/fagmed/norsk-gynekologisk-forening/veiledere/arkiv-utgatte-veiledere/veileder-i-fodselshjelp-2014/33.-cervixmodninginduksjon-av-fodsel Accessed 27.3.2025.
- 11.
Sørbye IK, Oppegaard KS, Weeks A et al. Induction of labor and nulliparity: A nationwide clinical practice pilot evaluation. Acta Obstet Gynecol Scand 2020; 99: 1700–9. [PubMed][CrossRef]
- 12.
Pettersen-Dahl A, Murzakanova G, Sandvik L et al. Maternal body mass index as a predictor for delivery method. Acta Obstet Gynecol Scand 2018; 97: 212–8. [PubMed][CrossRef]
- 13.
Dammer U, Bogner R, Weiss C et al. Influence of body mass index on induction of labor: A historical cohort study. J Obstet Gynaecol Res 2018; 44: 697–707. [PubMed][CrossRef]
- 14.
Vinturache A, Moledina N, McDonald S et al. Pre-pregnancy Body Mass Index (BMI) and delivery outcomes in a Canadian population. BMC Pregnancy Childbirth 2014; 14: 422. [PubMed][CrossRef]
- 15.
Lee VR, Darney BG, Snowden JM et al. Term elective induction of labour and perinatal outcomes in obese women: retrospective cohort study. BJOG 2016; 123: 271–8. [PubMed][CrossRef]
- 16.
Bjorklund J, Wiberg-Itzel E, Wallstrom T. Is there an increased risk of cesarean section in obese women after induction of labor? A retrospective cohort study. PLoS One 2022; 17: e0263685. [PubMed][CrossRef]
- 17.
Ellis JA, Brown CM, Barger B et al. Influence of Maternal Obesity on Labor Induction: A Systematic Review and Meta-Analysis. J Midwifery Womens Health 2019; 64: 55–67. [PubMed][CrossRef]
- 18.
Carlson NS, Hernandez TL, Hurt KJ. Parturition dysfunction in obesity: time to target the pathobiology. Reprod Biol Endocrinol 2015; 13: 135. [PubMed][CrossRef]
- 19.
Lassiter JR, Holliday N, Lewis DF et al. Induction of labor with an unfavorable cervix: how does BMI affect success? (‡). J Matern Fetal Neonatal Med 2016; 29: 3000–2. [PubMed][CrossRef]
- 20.
Marshall NE, Biel FM, Boone-Heinonen J et al. The Association between Maternal Height, Body Mass Index, and Perinatal Outcomes. Am J Perinatol 2019; 36: 632–40. [PubMed][CrossRef]
- 21.
Fattah C, Farah N, O'Toole F et al. Body Mass Index (BMI) in women booking for antenatal care: comparison between selfreported and digital measurements. Eur J Obstet Gynecol Reprod Biol 2009; 144: 32–4. [PubMed][CrossRef]
- 22.
Headen I, Cohen AK, Mujahid M et al. The accuracy of self-reported pregnancy-related weight: a systematic review. Obes Rev 2017; 18: 350–69. [PubMed][CrossRef]