A man in his fifties developed enterocolitis with subsequent electrolyte and protein loss, immunodeficiency and severe infections. The diagnosis is rare and serious.
A man in his fifties had previously been treated for recurrent bilateral iridocyclitis and scleritis with 17.5 mg of daily prednisolone tablets and 20 mg of weekly methotrexate injections. Four years prior to the current presentation, he was diagnosed with chronic lymphocytic leukaemia/small lymphocytic lymphoma after the incidental discovery of an enlarged lymph node in his neck. One year before the current presentation, a skin biopsy of recently developed pigmented plaques under his eyes revealed findings consistent with necrobiotic xanthogranuloma (Figure 1).
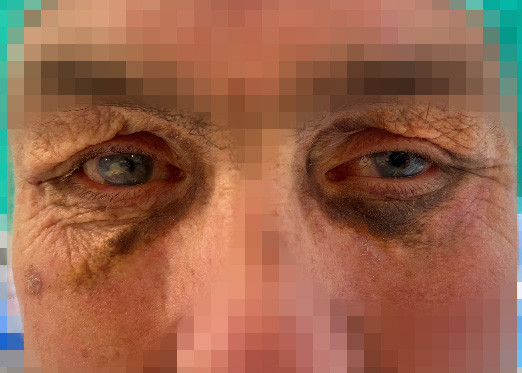
Necrobiotic xanthogranuloma is a non-Langerhans cell histiocytosis associated with lymphoproliferative diseases (1). Symptoms often include subcutaneous nodules or plaques in the skin, with up to 80 % of cases occurring in the periorbital region. Extracutaneous manifestations such as keratitis, uveitis and scleritis have been reported (1). No standardised treatment guidelines currently exist for the condition, and in most cases, treatment is aimed at underlying lymphoproliferative disease if present (2).
The patient's periorbital symptoms were considered secondary to his lymphoma, and treatment targeting the lymphoma was considered appropriate. Prior to starting treatment, a bone marrow biopsy revealed small foci with 90 % infiltration of chronic lymphocytic leukaemia, and flow cytometry showed 65 % chronic lymphocytic leukaemia cells. In addition, an M-component of the IgG-kappa type was detected at 0.4 g/L. Intravenous chemoimmunotherapy was initiated with rituximab 375 mg/m2 on day 1 and bendamustine 90 mg/m2 on days 1 and 2, with treatment planned every 28 days. At this point, methotrexate had been discontinued, and prednisolone had been tapered to 10 mg daily.
One month after starting chemoimmunotherapy, the patient was admitted to the local hospital with fever, vomiting and diarrhoea. He had no history of abdominal surgery, no known bowel disease, no family history of inflammatory bowel disease and no recent travel history. The patient denied using nonsteroidal anti-inflammatory drugs. Blood tests showed a C-reactive protein (CRP) level of 40 mg/L (reference range < 5), while the white blood cell count was within the normal range. After obtaining blood cultures, a urine sample and a stool sample, intravenous treatment was initiated with 3 g of benzylpenicillin four times daily and 6 mg/kg of gentamicin once daily.
Chemoimmunotherapy for lymphoma increases the risk of infections, typically bacterial. Patients are instructed to seek hospital care if they experience a temperature > 38.3°C from a single measurement or a persistent temperature > 38.0°C between treatments (3). Symptoms such as vomiting and diarrhoea, in combination with fever, do not necessarily indicate infections originating in the gastrointestinal tract, but may also be nonspecific (toxic) symptoms of sepsis.
The day after admission to hospital, a polymerase chain reaction (PCR) test was positive for norovirus in the stool, while blood cultures, stool cultures and urine cultures were sterile. The patient was afebrile with normal white blood cell levels and decreasing vomiting and diarrhoea. After four days, the antibiotics were discontinued, and the patient was discharged in a good state of health.
The patient was hospitalised three days later with a reduced general condition, abdominal pain and watery stools. Upon admission, he was afebrile with normal vital signs. Blood tests showed a CRP of 12 mg/L, normal leukocyte levels, creatinine at 59 µmol/L (reference range 60–105), estimated glomerular filtration rate (eGFR) of 108 mL/min and normal electrolytes. Blood cultures and stool samples were collected; the latter tested negative for Clostridioides difficile and for the gastrointestinal pathogen panel. Stool cultures for Aeromonas, Vibrio and Plesiomonas were also negative. The patient developed increasing lower abdominal pain, and a CT scan of the abdomen and pelvis was performed two days after admission, showing pronounced enterocolitis with inflammation throughout the small intestine and the right side of the colon (Figure 2). The following day, a faecal catheter was placed to relieve severe skin irritation around the anus, and the patient was given parenteral nutrition and electrolyte replacement. Four days after admission, a PCR test for cytomegalovirus (CMV) in EDTA plasma showed < 110 IU/mL (weak positive).
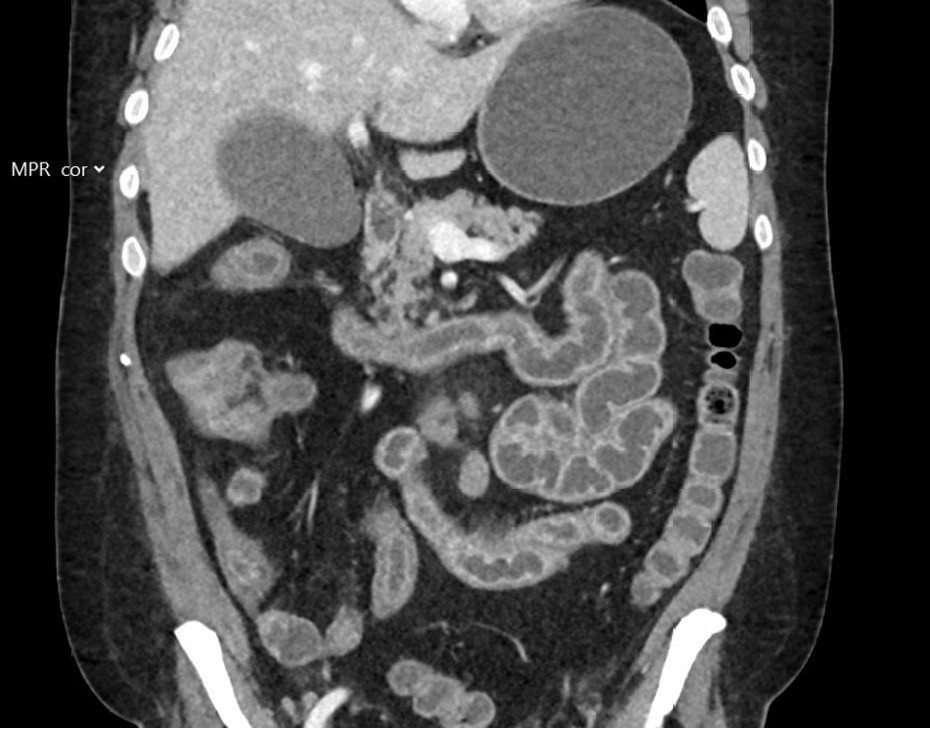
Cytomegalovirus is a commonly occurring herpes virus. The primary infection is asymptomatic in most immunocompetent patients, after which cytomegalovirus establishes itself as a latent infection with the potential for reactivation. In immunocompetent patients, reactivation is controlled by the immune system. In those with a suppressed immune system, cytomegalovirus can cause severe illness, both in primary infection and reactivation (4). CMV colitis is one of the most common manifestations, with abdominal pain, diarrhoea, fever, fatigue and weight loss being common symptoms (4, 5). The diagnosis is suspected based on a typical clinical picture and macroscopic findings from endoscopy (inflammation, oedema and ulcerations). It is confirmed by typical histopathological findings, such as large cells with inclusion bodies, and findings of CMV-infected cells with immunohistochemical staining (4). CMV DNA in biopsies and blood can supplement standard diagnostics but is not diagnostic on its own (4). CMV DNA can be detected in biopsies and blood in patients without CMV disease, and it may be absent in patients with the disease (4).
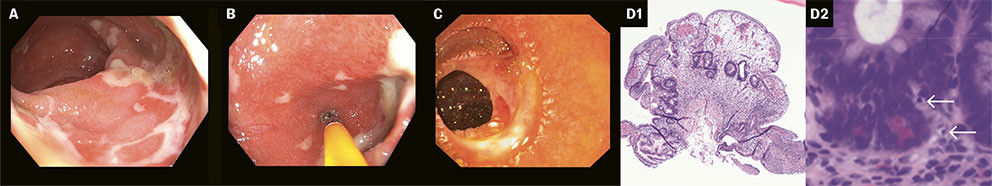
Seven days after admission, colonoscopy showed inflammation of the terminal ileum and oedematous mucosa, with blurring of vascular markings throughout the entire colon. There were also signs of deep ulcerations in the rectosigmoid junction (Figures 3a and 3b). Biopsies from the small and large intestines for microbiology and histology showed CMV DNA in the rectal biopsy. The condition was interpreted as CMV enterocolitis, and treatment was initiated with 5 mg/kg of intravenous ganciclovir × 2.
It is common to differentiate between CMV infection with CMV-DNA in blood or biopsy in asymptomatic patients and CMV disease with CMV-DNA in blood or biopsy in patients with concurrent clinical manifestations. Distinguishing CMV disease from other causes of colitis based on the endoscopic appearance and histological findings can be challenging. Reactivation of CMV with subsequent viremia can occur as a result of other inflammation, and CMV-DNA findings do not necessarily indicate an illness that requires treatment (4, 6). There is considerable variation between different PCR tests, and therefore no defined threshold for the amount of CMV-DNA that indicates disease (4).
Histopathological results from small and large intestine biopsies showed ulcerations, acute inflammation and reactive crypt changes. Immunohistochemical analysis of the rectum biopsy was CMV negative. There were no signs of CMV colitis due to the absence of inclusion bodies, nor any indications of inflammatory bowel disease. Ganciclovir was continued due to suspected CMV reactivation. The patient received total parenteral nutrition and had persistent diarrhoea with electrolyte disturbances and fibrin deposits in the faecal catheter system. Treatment with loperamide and cholestyramine had no effect on the diarrhoea, and use of opium tincture resulted in increased abdominal pain. Gastroscopy and upper balloon enteroscopy, performed two and a half weeks after admission, showed inflamed mucosa one metre from the pylorus and localised fibrotic bands (Figure 3c). Biopsies of the duodenal and jejunal mucosa revealed villous atrophy and acute inflammation, but immunohistochemistry was negative for cytomegalovirus and Helicobacter pylori, and both serology and histology tests for celiac disease were negative. A sample for PCR testing for Tropheryma whipplei was negative. CT scans of the chest, abdomen and pelvis performed 15 days after admission showed progressive enteritis in the jejunum, with wall thickening and fat stranding. There was no evidence of additional infection foci. After consulting with a gastroenterologist at the university hospital, 60 mg of intravenous methylprednisolone once daily was initiated.
Corticosteroids play an important role in the treatment of severe forms of inflammatory bowel disease, including chemotherapy-associated colitis and immune-mediated colitis (7).
The corticosteroid treatment had no marked effect on abdominal pain, diarrhoea or inflammatory markers, and was tapered over three weeks. Due to persistently elevated inflammatory markers, with no other microbiological findings except for a significant level of CMV-DNA in EDTA plasma (9,346 IE/mL), ganciclovir was continued. Two months after admission, the patient developed fever with a rectal temperature of 38.5°C, CRP of 100 mg/L with normal leukocytes and growth of E. coli in blood cultures but not in the urine. He was treated with intravenous piperacillin/tazobactam 4 g/0.5 g × 4 for ten days.
Two and a half months after admission, the patient developed severe hypoalbuminemia with albumin < 10 g/L (35–43) and oedema, which was treated with regular intravenous albumin infusions. There was no albuminuria, and the cause was presumed to be protein loss from the intestines in combination with catabolism due to reduced nutrient intake and inflammation. Because of the abdominal pain from tube feeding, there was a continued need for parenteral nutrition and the use of a pain pump. At this point, the patient had reduced renal function, with an eGFR of 40, based on a measurement of Cystatin-C at 1.67 mg/L (0.61–1.01). This was likely due to hypovolemia and prolonged treatment with ganciclovir.
Three months after admission, another consultation was held with a gastroenterologist at the university hospital. It was now suspected that the patient's treatment-refractory enterocolitis was caused by a previous rituximab infusion, and treatment with TNF-α inhibitors was suggested. In consultation with the patient and his family, 10 mg/kg of intravenous infliximab × 1 every 14 days was initiated. The patient's eye symptoms had ceased despite only one course of bendamustine and rituximab. After the treatment with infliximab, there was a gradual reduction in abdominal pain, falling inflammation markers and normalisation of stool consistency and colour.
Rituximab-induced enterocolitis is a rare and potentially serious condition for which the pathophysiology is not well understood. The condition can resemble inflammatory bowel disease both clinically and histologically (8). In the literature, the effects of rituximab discontinuation or treatment with corticosteroids, TNF-α inhibitors and colectomy are reported solely in case reports (9). As the patient had unsuccessfully tried corticosteroids previously and had extensive involvement of the small intestine, colectomy was not considered a viable option.
On the fifth day after treatment with infliximab, the patient again developed fever and rising inflammatory markers, with a CRP of 197 mg/L (< 5). Blood cultures showed growth of Enterococcus faecium, and intravenous vancomycin 20 mg/kg × 2 was initiated. Due to severe hypogammaglobulinemia with IgG 3.49 g/L (6.10–14.9) and recurrent infections, the patient was given intravenous immunoglobulin. Further treatment with infliximab was postponed due to the ongoing infection. After ten days of vancomycin, the patient was afebrile and had sterile blood cultures. A thorough investigation found no evidence of an infectious or hematologic cause for the patient's symptoms. A clinical and biochemical response was observed after the first dose of infliximab, and treatment was continued two weeks after the initial dose. The university hospital's re-examination of the biopsies from the intestinal mucosa revealed rituximab-induced enterocolitis (Figure 3d). No infectious agents were found with special staining. There was also no evidence of amyloidosis or lymphoma in the intestine, and examination with in situ hybridisation for Epstein-Barr virus was negative. Two weeks after the second dose, following a total of four months in the hospital, the patient again developed febrile symptoms and rising inflammatory markers, with Enterococcus faecium in the blood cultures. He was weak, somnolent, disoriented and had generalised oedema, progressive renal failure, severe hypernatremia and moderate hypokalaemia. Based on the finding of Pseudomonas aeruginosa in respiratory secretions, intravenous linezolid 600 mg × 2 and meropenem 1 g × 3 were initiated. Three days later, the patient was unresponsive with no signs of improvement, and, in consultation with his family, the decision was made to switch to palliative care. The patient passed away peacefully the following day. The autopsy report concluded with pronounced chronic inflammatory changes and ulcerations, consistent with rituximab-induced enterocolitis. Immunohistochemical examination detected a single CMV-positive cell in the small intestine, but it is uncertain what clinical relevance this finding has, as rituximab-induced enterocolitis was also detected. The intestine was likely the source of the deceased's sepsis. A report detailing the serious adverse effect of medication was submitted to the Norwegian Medical Products Agency, which confirmed a likely causal relationship between the fatal enterocolitis and rituximab.
Discussion
Rituximab is a monoclonal antibody that targets the transmembrane antigen CD20 found on B lymphocytes. It is used to treat non-Hodgkin's lymphoma and various autoimmune diseases. Rituximab is generally well tolerated, but adverse effects such as fever, nausea, vomiting and infections can occur (10).
Rituximab-induced enterocolitis is a rare condition, with fewer than 40 published cases worldwide. The mechanism of injury is not fully understood, but it has been suggested that it results from depletion of B lymphocytes in the intestinal wall, leading to secondary immune dysregulation and activation of T lymphocytes, causing intestinal inflammation (8, 9). A retrospective study from 2019 estimated the incidence of colitis among cancer patients treated with rituximab to be 4 %, with an average treatment duration of 99 days. The majority of the patients developed symptoms of colitis eight months after the first treatment (11). The study concluded that there was no clear correlation between colitis and an underlying immunosuppressive condition (11). An Icelandic study from 2021 showed that patients treated with rituximab – regardless of treatment indication, dose or duration – had a six times higher risk of developing inflammatory bowel disease compared to the general population (12). No association with concurrent autoimmune disease was found (12).
Rituximab-induced enterocolitis has not been reported following administration of just one dose of rituximab, and the authors believe that the treatment indication – chronic lymphocytic leukaemia/small lymphocytic lymphoma – was not, in itself, a contributing factor. Patients with underlying autoimmune disease, malignancy or prior immunosuppressive therapy have compromised immune systems, which in itself may increase the risk of immune dysregulation. This can make the intestinal mucosa more susceptible to inflammation or reactivation of latent infections in the gastrointestinal tract and potentially increase the risk of developing rituximab-induced enterocolitis (8, 9). It is difficult to distinguish between causality and coincidence, and to identify specific risk factors that predispose patients to rituximab-induced enterocolitis.
There are no established guidelines for managing the condition, but the literature describes cases where corticosteroids and TNF-α inhibitors have been used successfully. In some severe cases, colectomy and/or proctectomy have been necessary (8). Treatment with rituximab leads to immunosuppression, and further treatment with corticosteroids or TNF-α inhibitors increases the risk of opportunistic infections. Managing rituximab-induced enterocolitis is therefore a difficult balance between immunosuppressive therapy and the risk of infections (8).
The patient in the case history experienced a severe, treatment-refractory course of illness, where the diagnostic delay was primarily due to the symptoms initially being interpreted as CMV enterocolitis. It was subsequently concluded that CMV reactivation had occurred but there was no CMV disease. The symptoms appeared following chemoimmunotherapy for a haematological condition, but M-component levels were decreasing, and repeated CT scans revealed no enlargement or new development of lymph node tumours. As only a single course of chemoimmunotherapy had been administered shortly before symptom onset, an adverse reaction to medication was considered unlikely.
During the hospital stay, blood cultures were positive in three instances, which likely indicated bacterial translocation from the intestine due to impaired barrier function and a weakened immune system. It was only after reviewing the intestinal biopsies that evidence of rituximab-induced enterocolitis was found. Treatment with a TNF-α inhibitor had already been initiated and led to temporary improvement, but further treatment was complicated by severe infections, electrolyte imbalances, hypoalbuminemia, renal failure and malnutrition. Earlier treatment with infliximab might have reduced the risk of further immunosuppression caused by corticosteroids, which could have led to subsequent CMV reactivation and bacteraemia.
Conclusion
Rituximab-induced enterocolitis is a rare condition that has not previously been described in Norway. Use of rituximab in Norway has been increasing in recent years (13). Rituximab-induced enterocolitis should be considered if patients develop persistent abdominal pain and diarrhoea during treatment with rituximab.
The patient's family has consented to publication of the article.
The article has been peer-reviewed.
- 1.
Chakari W, Rangatchew F, Toyserkani NM et al. Necrobiotic xanthogranuloma misdiagnosed as a planocellular carcinoma. Ugeskr Laeger 2019; 181: V11180800. [PubMed]
- 2.
Miguel D, Lukacs J, Illing T et al. Treatment of necrobiotic xanthogranuloma - a systematic review. J Eur Acad Dermatol Venereol 2017; 31: 221–35. [PubMed][CrossRef]
- 3.
Helsedirektoratet. Lymfekreft - handlingsprogram. https://www.helsedirektoratet.no/retningslinjer/lymfekreft-handlingsprogram Accessed 28.10.2024.
- 4.
Dioverti MV, Razonable RR. Cytomegalovirus. Microbiol Spectr 2016; 4. doi: 10.1128/microbiolspec.DMIH2-0022-2015. [PubMed][CrossRef]
- 5.
Baniak N, Kanthan R. Cytomegalovirus Colitis: An Uncommon Mimicker of Common Colitides. Arch Pathol Lab Med 2016; 140: 854–8. [PubMed][CrossRef]
- 6.
Griffiths P, Reeves M. Pathogenesis of human cytomegalovirus in the immunocompromised host. Nat Rev Microbiol 2021; 19: 759–73. [PubMed][CrossRef]
- 7.
Schneider BJ, Naidoo J, Santomasso BD et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J Clin Oncol 2021; 39: 4073–126. [PubMed][CrossRef]
- 8.
Martinez Perez P, Hanna L, Jaynes E et al. Infliximab rescue therapy in a case of severe granulomatous colitis associated with rituximab use. BMJ Case Rep 2024; 17. doi: 10.1136/bcr-2023-257729. [PubMed][CrossRef]
- 9.
Lipka S, Katz S, Crawford JM. Fulminant Colitis Following Rituximab Therapy. Gastroenterol Hepatol (N Y) 2016; 12: 58–60. [PubMed]
- 10.
Gürcan HM, Keskin DB, Stern JNH et al. A review of the current use of rituximab in autoimmune diseases. Int Immunopharmacol 2009; 9: 10–25. [PubMed][CrossRef]
- 11.
Mallepally N, Abu-Sbeih H, Ahmed O et al. Clinical Features of Rituximab-associated Gastrointestinal Toxicities. Am J Clin Oncol 2019; 42: 539–45. [PubMed][CrossRef]
- 12.
Kristjánsson VB, Lund SH, Gröndal G et al. Increased risk of inflammatory bowel disease among patients treated with rituximab in Iceland from 2001 to 2018. Scand J Gastroenterol 2021; 56: 46–52. [PubMed][CrossRef]
- 13.
Folkehelseinstituttet. Legemiddelforbruket i Norge 2019-2023. https://www.fhi.no/contentassets/b0802ad9303347b682cf6a8fa701ec91/legemiddelforbruket-i-norge-2019-2023-rapport-2024.pdf Accessed 12.11.2024.