Main findings
Among 120 patients who underwent an allogeneic stem cell transplant for lymphoma during the periods 2011–16 and 2016–21, the proportion who died from transplant-related complications within two years, including graft-versus-host disease, decreased from 28 % to 15 %.
Among patients receiving a transplant in the period 2011–16, 22 % were alive with no relapse or graft-versus-host disease two years later, compared to 47 % of those who received a transplant in in the period 2016–21.
Allogeneic stem cell transplantation (ASCT) is potentially curative for patients with haematologic malignancies, lymphomas and certain non-malignant conditions. However, it carries a risk of severe complications and transplant-related mortality due to organ damage, infection and graft-versus-host disease (GVHD). The treatment is relevant for patients with lymphoma who experience a relapse or do not respond to standard treatment (1). For some patients with particularly severe disease characteristics, it may be considered as part of first-line treatment.
Lymphoma is a collective diagnosis for more than 30 different types of cancer that typically originate in the lymphoid organs. Symptoms can include enlarged lymph nodes, fever and weight loss. Lymphomas are divided into two main categories: Hodgkin's lymphoma and non-Hodgkin's lymphoma. Hodgkin's lymphoma often occurs in younger age groups and generally has a good prognosis. Non-Hodgkin's lymphoma is the largest group and consists primarily of B-cell lymphomas and T-cell lymphomas. Diffuse large B-cell lymphoma is the most common type, accounting for 30–40 % of cases. The choice of treatment depends on the type, extent and level of aggressiveness of lymphoma, and can involve a combination of chemotherapy, radiotherapy and immunotherapy. Survival rates for patients with lymphoma have improved significantly over the years due to better diagnostics, enhanced supportive care, intensified treatment for the most aggressive lymphomas and new medications (2). Chimeric antigen receptor T-cell therapy (CAR-T) has led to long-term survival or cure in some patients and a decrease in ASCT for patients with lymphoma (3).
ASCT is a form of immunotherapy in which the patient receives blood stem cells from either a family donor or an unrelated donor. The goal is for the donor's T lymphocytes to eliminate any remaining cancer cells and protect the patient from relapse. Before transplantation, the patient's own immune cells are weakened or destroyed from conditioning treatment, which involves chemotherapy on its own or in combination with radiotherapy. Before the transplant can proceed, the disease must be well controlled with induction therapy, which may include chemotherapy, radiotherapy or immunotherapy in various combinations.
The Department of Haematology at Oslo University Hospital serves as a national function for ASCT for adults with lymphoma. In the period 2011–2023, a total of 154 such patients underwent transplantation, accounting for 13 % of all transplant patients during this period. Transplant outcomes have previously been published in the Journal of the Norwegian Medical Association (4, 5). The study by Vo et al. includes all patients who underwent transplantation in the period 2015–2021 and demonstrates improvements in one-year survival rates and a reduction in complications during this period (5).
Some of the patients in our study were included in previous publications from our centre. However, each diagnostic group undergoing ASCT faces its own distinct challenges. Transplantation for lymphoma has been associated with a high incidence of complications (6). In this study, we specifically aimed to examine outcomes for patients with lymphoma.
GVHD is a severe and potentially life-threatening complication in which donor T lymphocytes attack the recipient's healthy tissues and organs. It is categorised as acute, which typically develops within 100 days, or chronic, which usually appears later than this. Both types can cause significant health problems and may be fatal. Affected organs can include the skin (manifesting as a rash), the eyes (causing dryness and inflammation) and the intestines (leading to ulcers and diarrhoea). All patients receive immunosuppressants to reduce the risk of GVHD. In October 2016, prophylaxis with antithymocyte globulin (ATG) was also introduced to further mitigate this complication. ATG is an antibody with immunosuppressive effects that reduce the number of T lymphocytes involved in the immune response. At the correct dosage, it lowers the risk of GVHD and transplant-related mortality without increasing the risk of relapse (7). A study from our centre demonstrated that transplant-related mortality decreased from 33 % to 17 %. Additionally, the likelihood of surviving without GVHD or relapse after three years increased from 43 % to 64 % when ATG was administered to patients with haematologic malignancies who underwent transplantation with stem cells harvested from peripheral blood (8).
In this study, we compared two groups of patients with lymphoma who underwent transplantation before and after the introduction of ATG prophylaxis. All patients were followed for at least two years, and we have chosen to present data from this two-year period.
Material and method
This is a retrospective registry study that includes patients who underwent their first ASCT for lymphoma at the Department of Haematology, Oslo University Hospital, between 1 January 2011 and 30 September 2021. One patient was under the age of 18 and the others were 18 years or older. The different lymphoma types are presented in Table 1. Patients with lymphoma in combination with other cancer diagnoses or immunodeficiency were excluded. Data were obtained from the department's quality register, with links to the National Population Register.
Table 1
Lymphoma type, two-year survival and relapse-free survival for 120 patients who underwent ASCT at Oslo University Hospital in the period 1 January 2011–30 September 2021
| Lymphoma type | No. of patients group | Two-year survival as a % | Two-year relapse-free survival as a % (95 % confidence interval) | |
|---|---|---|---|---|
| Hodgkin's lymphoma | 10 | 70 (33–89) | 40 (12–67) | |
| B-cell lymphomas: | ||||
| Diffuse large B-cell lymphoma | 22 | 55 (32–72) | 55 (32–72) | |
| Follicular lymphoma | 18 | 61 (35–79) | 56 (31–75) | |
| Mantle cell lymphoma | 27 | 89 (69–96) | 74 (53–87) | |
| Transformed follicular lymphoma | 11 | 64 (30–85) | 64 (30–85) | |
| Other/mixed lymphomas | 6 | - | - | |
| T-cell lymphomas: all subcategories | 26 | 58 (37–74) | 39 (20–56) | |
The patients were divided into two groups: group 1 (65 patients) was transplanted in the period 1 January 2011–30 September 2016; group 2 (55 patients) was transplanted in the period 1 October 2016–30 September 2021. Data retrieval and censoring were carried out on 24 January 2024. All patients were followed for at least two years, or until their death if this occurred before the two-year mark. We recorded survival, relapse-free survival and transplant-related mortality. Transplant-related mortality was defined as all non-relapse deaths, and included deaths from GVHD, multiorgan failure, infection, coronary disease, post-transplant lymphoma, stroke and haemorrhage. Relapse-free patients who were alive at the last follow-up were censored. We recorded acute and chronic GVHD. Acute GVHD is graded from I–IV, with III and IV characterised as severe, i.e. significantly affecting quality of life and with a risk of death. We recorded chronic GVHD for patients who lived > 100 days. This is graded as mild, moderate or severe. All grades cause health issues, and severe grades can be fatal. Survival without GVHD or relapse was recorded: events were defined as the first occurrence of grade III–IV acute GVHD, moderate or severe chronic GVHD, relapse or death. Patients with no recorded events at the last follow-up were censored.
Statistical analyses
Categorical variables are presented as numbers and percentages, while continuous variables are presented as median values with the lowest and highest observed values. The Kaplan-Meier estimator was used to calculate survival probability, relapse-free survival and survival without relapse or GVHD. Cumulative incidence of treatment-related mortality, GVHD and relapse was estimated using the Fine-Gray model (9). Analyses were performed using Statistica, version 14.0 (TIBCO, CA, USA) and EZR, version 1.61 (Saitama Medical Centre, Japan).
Ethics
The registry was approved by the hospital's data protection officer and permission to publish was granted by the Regional Committees for Medical and Health Research Ethics (REK) (reference 11909/2021).
Results
Patients
Patient characteristics are shown in Table 2. The median follow-up time was 101 months (30–145) in group 1 and 57 months (28–83) in group 2. The median age at the time of transplantation was 57 years (16–66) and 61 years (31–72), respectively. In the entire group, the most commonly occurring B-cell lymphomas were mantle cell lymphoma (27 patients) and diffuse large B-cell lymphoma (22 patients). A total of 26 patients had T-cell lymphoma, and 10 patients had Hodgkin's lymphoma (Table 1).
Table 2
Patient and donor characteristics, stem cell sources, prophylaxis for GVHD and conditioning treatment for 120 patients with lymphoma who underwent ASCT at Oslo University Hospital in the period 1 January 2011–30 September 2016 (group 1) and 1 October 2016–30 September 2021 (group 2).
| Variable | Group 1 | Group 2 | |
|---|---|---|---|
| Number of patients | 65 | 55 | |
| Age at time of transplantation, years, median (range) | 57 (16–66) | 61 (31–72) | |
| Sex | |||
| Male (number) | 47 | 39 | |
| Female (number) | 18 | 16 | |
| Donor's age, years, median (range) | 41 (19–72) | 26 (18–70) | |
| Donor type, number | |||
| Full-match family donor | 23 | 6 | |
| Unrelated donor | 40 | 48 | |
| HLA-haploidentical donor1 | 2 | 1 | |
| Stem cell source, number | |||
| Bone marrow | 3 | 1 | |
| Blood | 62 | 54 | |
| Prophylaxis for GVHD, number | |||
| Antithymocyte globulin | 0 | 48 | |
| Cyclosporine and sirolimus | 54 | 43 | |
| Other medications | 11 | 12 | |
| Pre-transplant conditioning treatment, number | |||
| Bone marrow ablation | 2 | 1 | |
| Reduced intensity | 63 | 54 | |
1 A family donor who shares one haplotype (a collection of genes inherited from one of the parents) with the patient.
Transplant process
Characteristics of the donor, stem cell sources, conditioning treatment and prophylaxis for GVHD are presented in Table 2. A total of 117/120 patients received reduced-intensity conditioning treatment immediately prior to transplant, while 3 received more intensive, myeloablative conditioning. In group 2, 48/55 patients received ATG in addition to other immunosuppressants.
Survival, causes of death and relapse
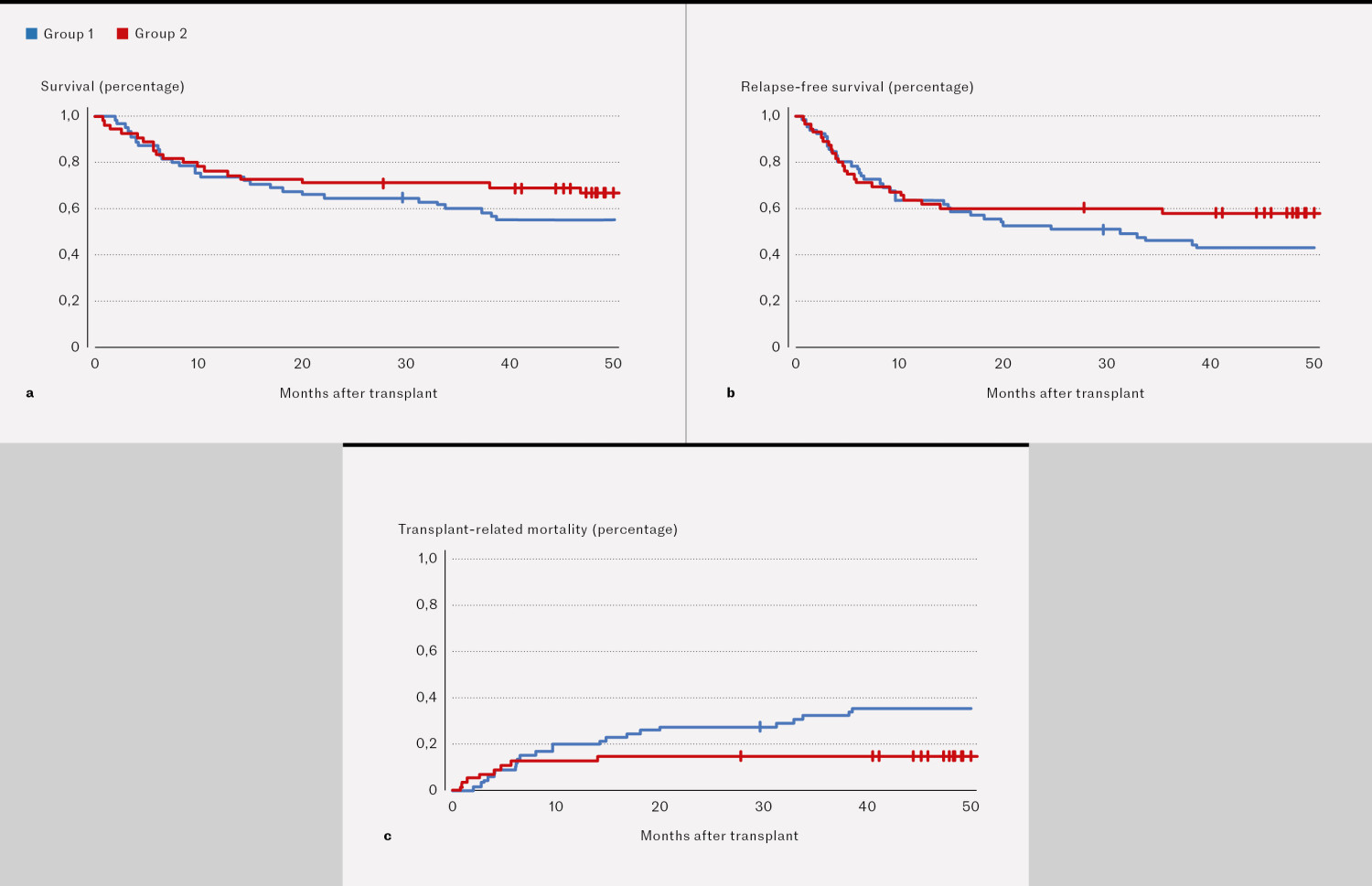
Two-year survival was 42/65 (65 %) in group 1 and 39/55 (71 %) in group 2 (Figure 1a). Two-year relapse-free survival was 34/65 (52 %) and 33/55 (60 %), respectively (Figure 1b). Transplant-related mortality was 18/65 (28 %) in group 1 and 8/55 (15 %) in group 2 (Figure 1c). A total of 5/65 patients in group 1 and 7/55 in group 2 died following a relapse. The most common causes of death in group 1 were GVHD, multiorgan failure and relapse, with 5 patients dying in each of these categories. A total of 8/23 patients died from other transplant-related causes. In group 2, 7/16 patients died following a relapse, while 8 died from a range of transplant-related causes. In one patient, the cause of death was unknown. Survival and relapse-free survival for the different lymphoma types are shown in Table 1.
GVHD
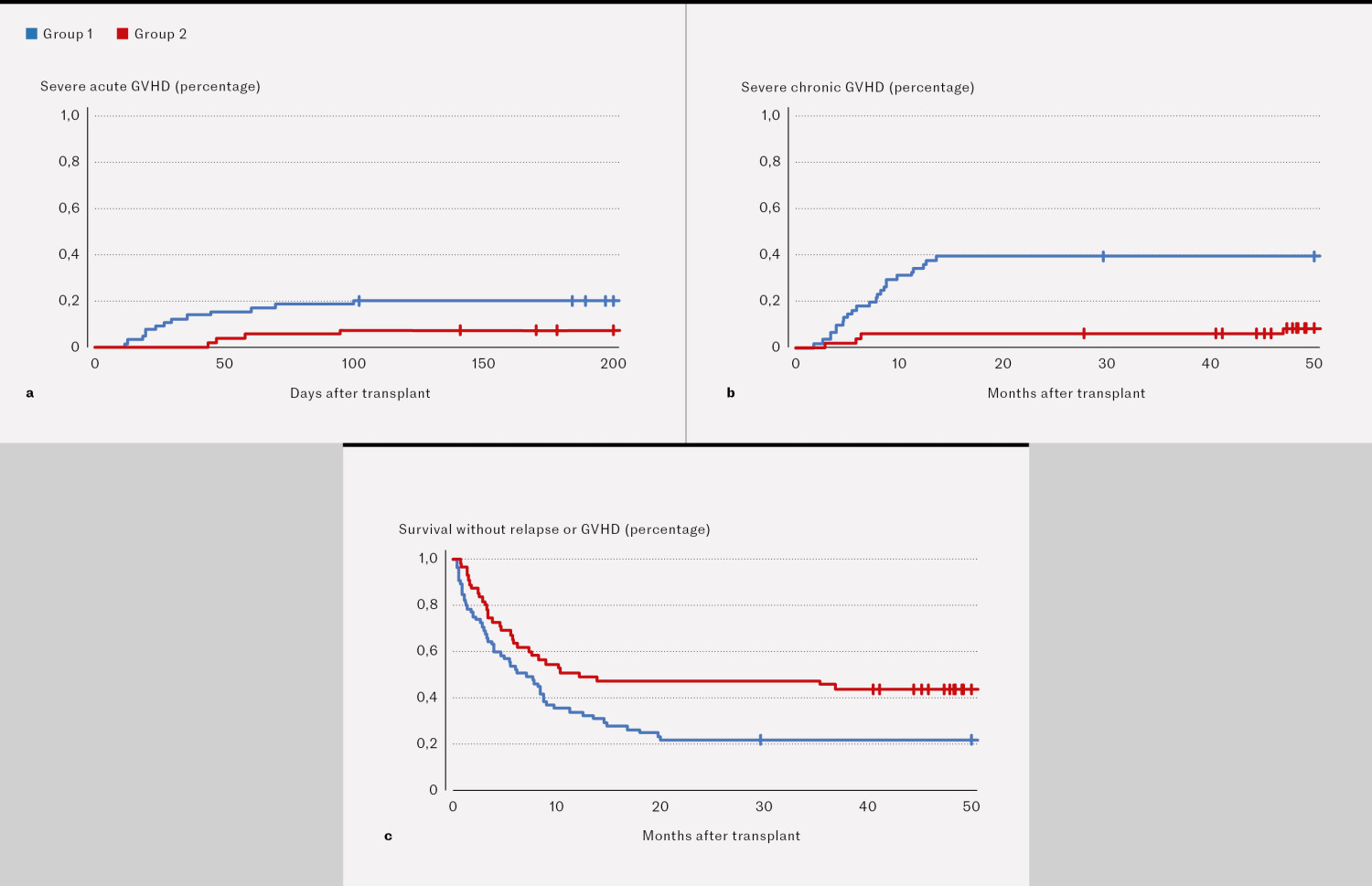
After two years, 13/65 patients (20 %) in group 1 and 4/55 patients (7 %) in group 2 had developed severe acute GVHD (Figure 2a). Of the patients who survived for at least 100 days, 24/61 (39 %) in group 1 and 3/51 (6 %) in group 2 developed severe chronic GVHD after two years (Figure 2b). At two years, 14/65 patients (22 %) in group 1 and 26/55 patients (47 %) in group 2 had survived without relapse or GVHD (Figure 2c).
Discussion
In our study of 120 patients who underwent transplantation for various types of lymphoma in the period 2011–2021, we found a significant reduction in transplant-related mortality and in the incidence of GVHD after two years for patients transplanted in the period 2016–21 compared to 2011–16. The proportion of patients who developed se<vere acute GVHD decreased from 20 % to 7 %, and the proportion who developed severe chronic GVHD decreased from 39 % to 6 % in the two time periods. The reduction in post-transplant complications and transplant-related mortality over time is in line with our own and others' experiences (5, 10–13).
Survival without relapse or GVHD is the desired outcome after ASCT. This is considered a good surrogate marker for successful treatment outcome, and means that the patient survives without relapse and without GVHD that impacts on their quality of life. In group 1, 22 % achieved this, compared to 47 % in group 2, which represents a clinically significant improvement.
The main difference in the treatment approach between the two time periods is that the majority of patients in the second group received prophylaxis with ATG in addition to standard immunosuppressants. This contributed to a significant reduction in the proportion of patients who developed severe GVHD and who died from this complication. However, changes were made to the transplant programme throughout the period 2011–21, which has likely also contributed to improved outcomes: Each transplant process is tailored to the individual patient. The stem cell donor's tissue type is carefully matched to the patient, and there is an increasing use of young, unrelated donors instead of older family donors. The pre-transplant conditioning treatment is also individualised and takes into account both the desired effect on the cancer and the patient's expected tolerance.
Overall mortality does not appear to be lower in the group transplanted in 2016–21 compared to 2011–16, but there is a trend towards lower mortality in the second group, which we believe could become significant with a larger sample size and/or longer follow-up period. In the second group, a few more patients died following a relapse, but there are insufficient numbers to draw any conclusions. This group has a lower risk of serious complications and transplant-related mortality, and a larger proportion of patients have a better quality of life, before some experience a relapse. Relapse remains a significant challenge in ASCT for malignant diseases, and the prognosis after relapse is generally poor.
When comparing outcomes across all lymphoma types over the entire period, patients with mantle cell lymphoma had the best results, with 89 % alive and 74 % remaining relapse-free after two years (Table 1). For mantle cell lymphoma, most relapses are expected within two years, but they can also occur after this (14). Patients with T-cell lymphoma have the poorest outcomes: 58 % were alive after two years, and 39 % were relapse-free. The outcomes are roughly in line with other studies (15). There are few patients in each diagnostic group, and the results have wide confidence intervals. However, we believe that the figures align well with clinical experience and provide a relatively accurate picture of the post-transplant outcome for different types of lymphoma.
A transplant can only be performed if the disease is well-controlled. We do not have detailed information about the disease status at the time of transplantation for each patient, but for the most aggressive lymphomas, such as diffuse large B-cell lymphoma, there is a general requirement for complete remission, meaning the patient must be disease-free. For the less aggressive, so-called indolent lymphomas, such as follicular lymphoma, at least partial response/partial remission is required. While we cannot draw any definitive conclusions about the impact of remission status on our patients' outcomes, a restrictive approach requiring good disease control has likely contributed to the good treatment outcomes.
All ASCTs for patients with lymphoma are performed at Oslo University Hospital, and the outcomes are considered representative for patients undergoing such treatment. However, these patients constitute a selected group in which good disease control has been achieved, and who are otherwise deemed fit to tolerate such intensive treatment.
Data are continuously entered into our registry by dedicated staff, and the recording of complications and deaths is considered to have been consistent throughout the period.
Conclusion
In recent years, several new treatment options have become available for patients with lymphoma. Proper application of ASCT requires knowledge about complications and expected effects. The study shows that the risk of serious transplant-related complications was significantly lower for patients transplanted in 2016–21 compared to 2011–16. However, post-transplant relapse remains a challenge. The treatment appears to be a good option for selected patients.
The article has been peer-reviewed.
- 1.
Lymfekreft handlingsprogram: https://www.helsedirektoratet.no/retningslinjer/lymfekreft-handlingsprogram Accessed 15.8.2024.
- 2.
Kreftregisteret. Nasjonalt kvalitetsregister for lymfoide maligniteter. Resultater og forbedringstiltak fra Nasjonalt kvalitetsregister for lymfoide maligniteter. Årsrapport 2023. https://www.kreftregisteret.no/globalassets/publikasjoner-og-rapporter/arsrapporter/publisert-2024/arsrapport-2023-nasjonalt-kvalitetsregister-for-lymfoide-maligniteter.pdf Accessed 15.1.2025
- 3.
Passweg JR, Baldomero H, Ciceri F et al. Hematopoietic cell transplantation and cellular therapies in Europe 2022. CAR-T activity continues to grow; transplant activity has slowed: a report from the EBMT. Bone Marrow Transplant 2024; 59: 803–12. [PubMed][CrossRef]
- 4.
Husøy MAR, Brinch L, Tjønnfjord GE et al. Allogen stamcelletransplantasjon hos voksne 1985-2012. Tidsskr Nor Laegeforen 2014; 134: 1569–75. [PubMed]
- 5.
Vo CD, Myhre AE, Abrahamsen IW et al. Allogen stamcelletransplantasjon hos voksne 2015–21. Tidsskr Nor Legeforen 2023; 143. doi: 10.4045/tidsskr.22.0521. [PubMed][CrossRef]
- 6.
Fløisand Y, Brinch L, Gedde-Dahl T et al. Ultra-short course sirolimus contributes to effective GVHD prophylaxis after reduced-intensity allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2012; 47: 1552–7. [PubMed][CrossRef]
- 7.
Cell Therapy Transplant Canada. Addition of anti-thymocyte globulin to standard graft-versus-host disease prophylaxis versus standard treatment alone in patients with haematological malignancies undergoing transplantation from unrelated donors: final analysis of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol 2020; 7: e100–11. [PubMed][CrossRef]
- 8.
Ali MM, Grønvold B, Remberger M et al. Addition of Anti-thymocyte Globulin in Allogeneic Stem Cell Transplantation With Peripheral Stem Cells From Matched Unrelated Donors Improves Graft-Versus-Host Disease and Relapse Free Survival. Clin Lymphoma Myeloma Leuk 2021; 21: 598–605. [PubMed][CrossRef]
- 9.
Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc 1999; 94: 496–509. [CrossRef]
- 10.
McDonald GB, Sandmaier BM, Mielcarek M et al. Survival, Nonrelapse Mortality, and Relapse-Related Mortality After Allogeneic Hematopoietic Cell Transplantation: Comparing 2003-2007 Versus 2013-2017 Cohorts. Ann Intern Med 2020; 172: 229–39. [PubMed][CrossRef]
- 11.
Remberger M, Ackefors M, Berglund S et al. Improved survival after allogeneic hematopoietic stem cell transplantation in recent years. A single-center study. Biol Blood Marrow Transplant 2011; 17: 1688–97. [PubMed][CrossRef]
- 12.
Penack O, Peczynski C, Mohty M et al. How much has allogeneic stem cell transplant-related mortality improved since the 1980s? A retrospective analysis from the EBMT. Blood Adv 2020; 4: 6283–90. [PubMed][CrossRef]
- 13.
Cooper JP, Storer BE, Granot N et al. Allogeneic hematopoietic cell transplantation with non-myeloablative conditioning for patients with hematologic malignancies: Improved outcomes over two decades. Haematologica 2021; 106: 1599–607. [PubMed][CrossRef]
- 14.
Lew TE, Cliff ERS, Dickinson M et al. Allogeneic stem cell transplantation achieves long-term remissions in mantle cell lymphoma, including in TP53-mutated disease. Leuk Lymphoma 2023; 64: 1792–800. [PubMed][CrossRef]
- 15.
Singh V, Kim S, Deol A et al. Allogeneic hematopoietic stem cell transplantation in T-cell lymphoma: a Meta-Analysis. Leuk Lymphoma 2022; 63: 855–64. [PubMed][CrossRef]