A woman in her thirties with dyspnoea two weeks after abdominoplasty
Two weeks after undergoing abdominoplasty, a woman was hospitalised due to dyspnoea, reduced general condition and elevated D-dimer. It transpired that the patient had a relatively common condition but with rare manifestations.
The patient was a woman in her thirties with previously untreated multiple sclerosis. She was taking 50 mg mirabegron extended-release tablets daily for urge incontinence. Two weeks earlier, she had undergone abdominoplasty with liposculpture after pregnancy, for which she took a three-day course of thrombosis prophylaxis with low molecular weight heparin.
She was admitted to the emergency department with reduced general condition and dyspnoea two weeks after surgery. She was awake and alert, oxygen saturation was 87–91 % without oxygen supplementation, blood pressure 113/69 mmHg, respiratory rate 22–24 per minute and pulse 97 beats/min. She developed a fever (38.7 °C) after admission, but findings from other organ examinations were normal, and ECG showed sinus rhythm. D-dimer at the emergency clinic was elevated at 31.9 mg/L (reference range < 0.50). Additional tests conducted in the emergency department showed mild anaemia, with haemoglobin (Hb) 11.2 g/dL (11.7–15.3), elevated C-reactive protein (CRP) 30 mg/L (< 5) and lactate dehydrogenase (LD) 316 U/L (105–205), and elevated cardiac biomarkers in the form of cardiac troponin T 42 ng/L (< 15). Arterial blood gas analysis on admission showed hypoxemia with pO2 7.9 kPa (11.1–14.4) and respiratory alkalosis with pH 7.47 (7.35–7.45), pCO2 4.3 kPa (4.7–5.9), base excess (BE) −0.3 mmol/L (0±3) and bicarbonate (HCO3-) 23 mmol/L (22–26).
Based on the patient's symptoms, clinical findings and elevated D-dimer, pulmonary embolism was the primary working diagnosis. Elevated cardiac troponin T can be seen in, inter alia, larger pulmonary emboli with increased myocardial strain.
Based on the working diagnosis, CT pulmonary angiography was performed, revealing pulmonary emboli in the right main artery and in segmental and subsegmental pulmonary arteries bilaterally (Figure 1). In both the right and left lung, basal, peripheral, wedge-shaped pulmonary opacities indicative of lung infarctions were observed. Mild dilation of the pulmonary trunk of 34 mm was also revealed, further supporting the suspicion of elevated pulmonary pressure and right ventricular strain.
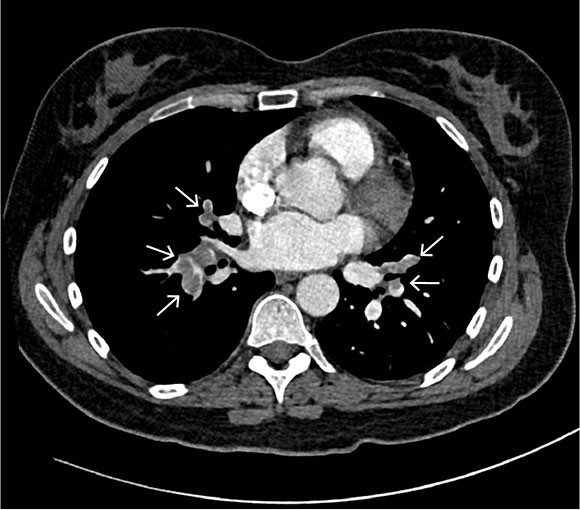
In Norway, venous thromboembolism is a common cause of hospital admission, and the annual incidence is estimated at 1.43/1000 (1). Many risk factors have been identified for venous thromboembolic disease, including increasing age, recent surgery, pregnancy, use of peroral oestrogen-containing contraceptives, prolonged immobilisation, obesity, smoking, infectious diseases, malignant diseases, chemotherapy as well as hereditary or acquired thrombophilias such as antiphospholipid syndrome (2).
The patient was treated with low molecular weight heparin in the form of a total of 15,000 IU dalteparin subcutaneously for pulmonary embolism. Due to elevated cardiac troponin T and signs of right ventricular strain on CT angiography, she was monitored by telemetry and further referred for a transthoracic echocardiogram to assess the right heart chamber. This showed a normally sized well-functioning right ventricle, but with signs of right-sided pressure overload based on a slightly elevated systolic pulmonary artery pressure of 42 mmHg (< 35–40) and a flattened interventricular septum. Left ventricular systolic function was normal, with an ejection fraction of 65 %.
More than 24 hours after admission, the patient experienced acute onset of pain in the right thorax and abdomen, with the point of maximal intensity in the right iliac fossa, accompanied by diffuse tenderness on percussion in the lower quadrant and periumbilical area. Blood tests showed an increase in CRP to 61 mg/L and a slight decrease in Hb to 9.9 g/dL.
Kidney function and liver tests were normal. ECG showed no signs of myocardial ischemia, and there was no suspicion of systemic infection. The patient was seen by a gastroenterology surgeon, who recommended an abdominal CT for diagnostic clarification.
Acute pain in the hemithorax and right flank of the abdomen can have a multitude of causes, with kidney stones, appendicitis, diverticulitis, gallstones, urinary tract infection, bleeding and acute gynaecological problems being the main differential diagnoses.
The abdominal CT with intravenous contrast showed well-demarcated, peripheral, wedge-shaped hypodense areas in the right kidney, most consistent with recent renal infarction (Figure 2). No bleeding or other relevant pathology was observed in the abdomen.
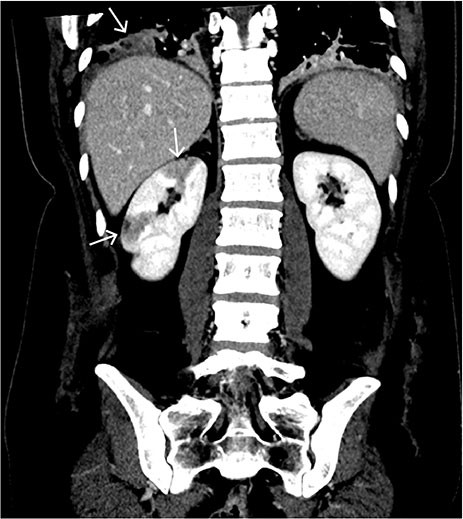
Hypodense lesions in the renal parenchyma can be seen in hypoperfusion and are mostly caused by acute renal infarction or pyelonephritis. Due to the well-demarcated peripheral, wedge-shaped lesions and absence of reaction in the perirenal adipose tissue, the findings are most consistent with renal infarction. This is a rare condition in which complete or partial occlusion of the renal artery or its branches causes ischemia in the kidney. Renal infarction can present with flank pain, nausea, fever and haematuria (3). However, the clinical picture can be subtle and the diagnosis can be missed. The condition can be complicated by reduced glomerular filtration and kidney failure.
There are no robust studies on the prevalence of the condition, but a study from 2006 of 18,287 patients with hypertension reported a prevalence of 0.3 % and an annual incidence of 0.018 % for spontaneous renal infarction, while another report from 1940 based on 14,411 autopsies showed a prevalence of 1.4 % (4, 5). The most common cause of renal infarction is cardiac thromboembolism, primarily due to atrial fibrillation (6). Other causes include renal artery injury and hypercoagulable states. There is no consensus on the treatment and follow-up for renal infarction, but catheter-based and systemic thrombolysis, anticoagulant therapy and surgery may be appropriate. Follow-up should include measuring kidney function and examination for signs of kidney damage (proteinuria and haematuria) and complications (7).
Transthoracic echocardiography performed earlier during the hospital stay had not shown intracardiac thrombus. Paradoxical embolisation was now the suspected source of the renal infarction, and transoesophageal echocardiography was therefore performed to look for signs of patent foramen ovale between the right and left atria of the heart. The patient was also referred for a brain MRI and fundoscopic examination to assess for further embolisation to other organs.
The transoesophageal echocardiogram showed patent foramen ovale with a tunnel length of 10 mm and an opening of 5 mm (Figure 3a), and a hypermobile atrial septum. Spontaneous right-to-left shunt of arterial emboli was observed using colour Doppler (Figure 3b).
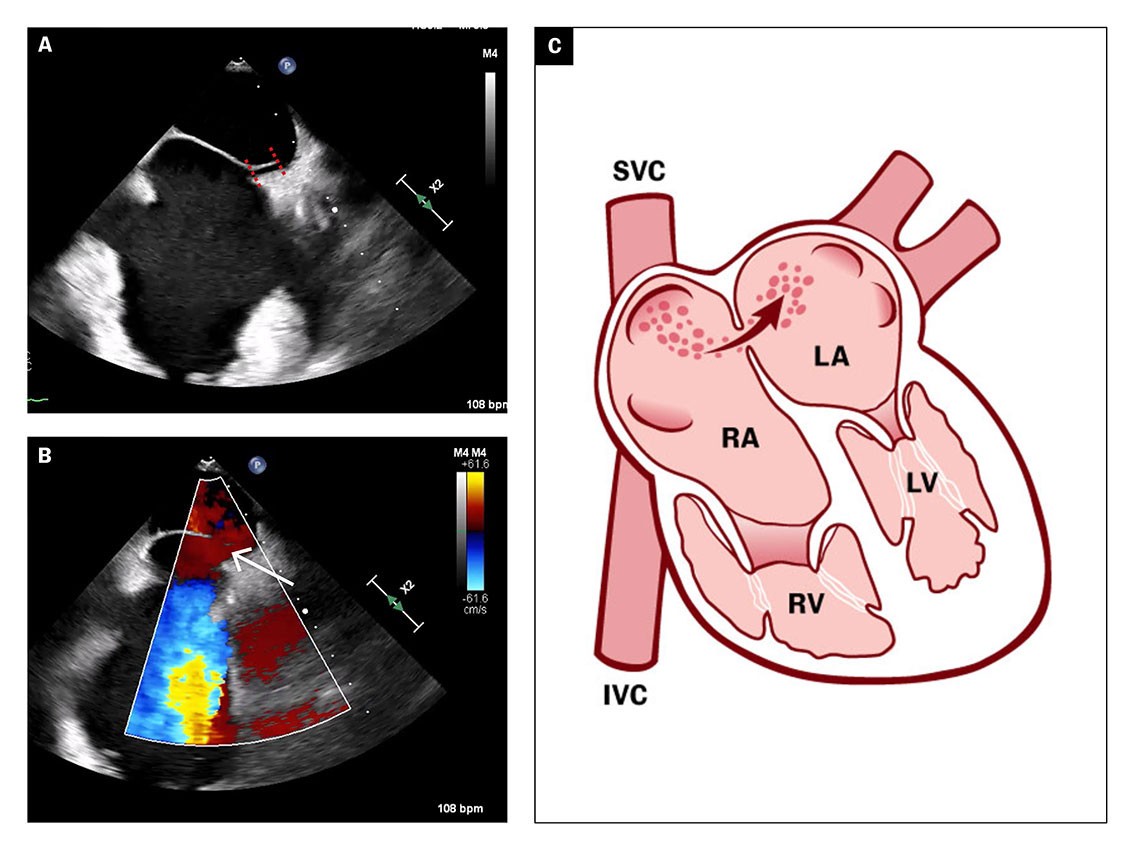
The transoesophageal echocardiogram findings supported the suspicion of paradoxical embolisation with right-to-left shunt of atrial thrombus. Patent foramen ovale is usually verified by peripherally injected agitated saline and the Valsalva manoeuvre. Increased venous return to the right atrium would then cause a temporary increase in atrial pressure, which can lead to right-to-left atrial passage of microbubbles (Figure 3c).
Due to a clear spontaneous right-to-left shunt, no bubble test was performed on this patient. She was assessed for percutaneous catheter-based closure of the patent foramen ovale to reduce the risk of future arterial embolisation. As part of the investigation, an ultrasound of the deep veins was also performed. This revealed bilateral thrombi in the leg veins which were described as mobile and with a chronic appearance. A brain MRI and fundoscopic examination did not reveal evidence of recent cerebral or retinal embolic infarction.
After an eight-day hospital stay, the patient was discharged with anticoagulant therapy in the form of peroral apixaban tablets and referred for elective catheter-based closure of the patent foramen ovale. Balloon measurement of the patent foramen ovale revealed a short tunnel with a diameter of 15 mm, which was closed percutaneously with a 30 mm GORE Septal Occluder (Figures 4a–b), a self-expanding implant made of nitinol and GORE-TEX, with two surfaces that enable closure of the atrial septum on both sides (Figure 4c).
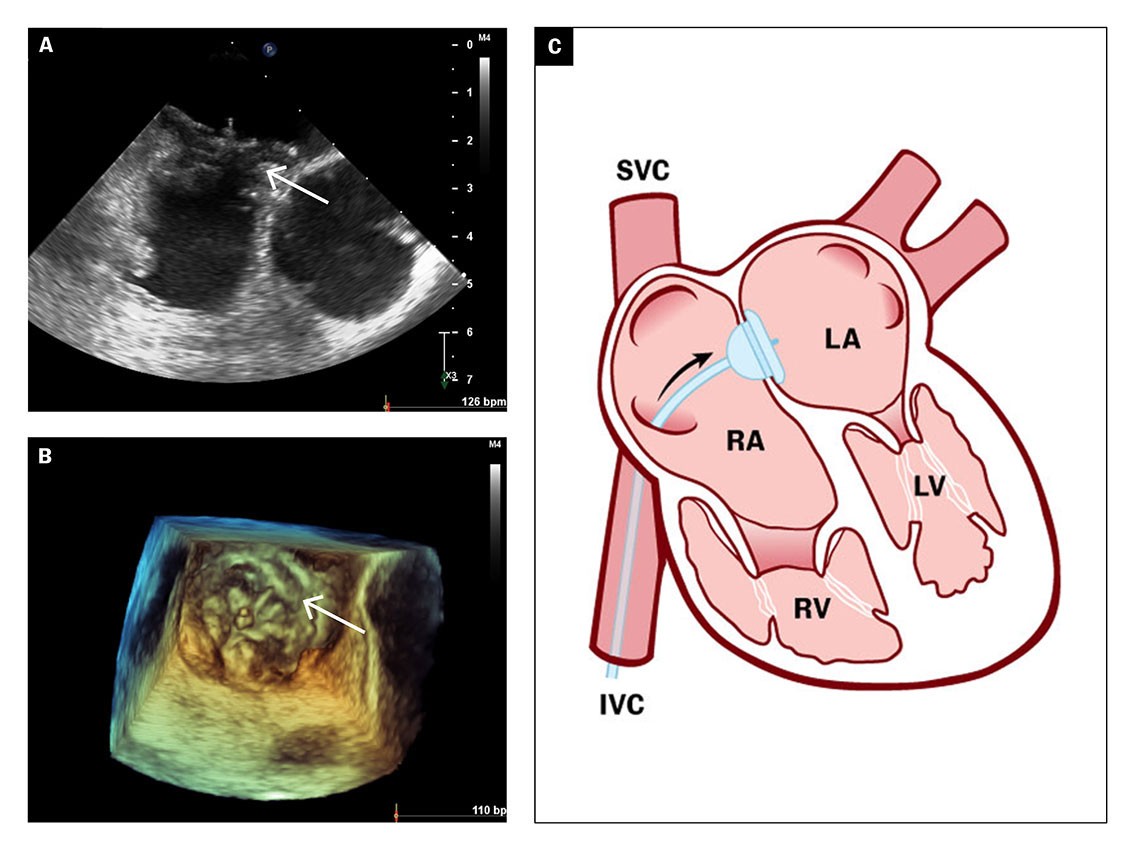
The implant is normally covered by endothelium within 3–6 months. The patient was considered to have a low risk of venous thromboembolism recurrence, and apixaban tablets were discontinued three months after closure. She also received acetylsalicylic acid 75 mg × 1 tablet for six months. A routine outpatient check-up with transoesophageal echocardiography was carried out ten weeks and then twelve months after the procedure, both of which showed successful closure of foramen ovale with the implant properly positioned and no right-to-left atrial passage of contrast during the Valsalva manoeuvre. The patient has not had any new cardiovascular events.
Anticoagulant therapy, as given to our patient, would also be preferable for thromboembolic aetiology of the relevant renal infarction. Our patient had no definite sequelae at subsequent check-ups. The duration of anticoagulant therapy will depend on the aetiology of venous thromboembolism. For intercurrent causes, such as surgery in our patient's case, a six-month course of anticoagulant therapy is recommended.
The patient's kidney function has been normal at all subsequent check-ups.
Discussion
Our patient had bilateral pulmonary emboli, likely originating from deep vein thrombosis following recent surgery, complicated by renal infarction caused by paradoxical embolisation via patient foramen ovale in the heart. Increased pressure in the right heart chamber due to pulmonary emboli likely caused intracardiac right-to-left shunting of the thrombus.
Patent foramen ovale occurs in up to 25 % of the general population (8, 9). Julius Friedrich Cohnheim, a German pathologist, described the association between patent foramen ovale and stroke in 1877 (10, 11). In fetal circulation, the foramen ovale serves as a vital aperture in the septum between the atria of the heart, which normally closes during the first year of life. However, incomplete closure allows for shunting between the right and left atrium.
Although most patients with patent foramen ovale are asymptomatic, the condition is associated with an increased risk of stroke, migraine attacks with aura, decompression sickness in divers and dyspnoea related to platypnea-orthodeoxia syndrome (12). In stroke patients under the age of 60 with no other apparent underlying cause, patent foramen ovale is much more common than in the general population, with an incidence of up to 50 %. A causal relationship is therefore assumed to exist between patent foramen ovale and stroke in these patients (13). Patients with patent foramen ovale can in practice experience emboli in all vascular organ systems, typically in the brain, eyes, spleen and gastrointestinal organs. However, the literature only describes a few cases of paradoxical emboli as a cause of renal infarction (14), usually in a scenario where several organs are affected by acute ischemic symptoms.
The gold standard in the investigation of patients with suspected patent foramen ovale is transoesophageal echocardiography using saline contrast injected into a peripheral vein. This is followed by visualisation of the passage of bubble contrast through the patent foramen ovale after the Valsalva manoeuvre (15).
No scientific evidence had previously been found of surgical closure being preferable to antithrombotic therapy as secondary prevention after stroke (16). It was not until 2017 that it was reliably documented that percutaneous closure of patent foramen ovale was a safe and effective modality that reduced the risk of recurrent stroke. Three independent randomised clinical trials were subsequently published, showing a significantly lower risk of new clinical stroke following closure combined with antithrombotic therapy compared to antithrombotic therapy alone (17–19). Consequently, the number of foramen ovale closure procedures performed has increased considerably, and more patients will also be spared long-term antithrombotic therapy.
Today, there is a well-documented risk-reducing effect of closing patent foramen ovale in patients who are presumed to have experienced an embolic stroke before the age of 60 without any other known cause. Closure is recommended for this group in accordance with national and international guidelines (20). The studies did not include patients with embolisation to organs other than the brain, but a corresponding reduction in risk of new emboli must be assumed for all patients with documented embolisation in the systemic circulation. In our patient, where patent foramen ovale was the likely cause of renal infarction, we found an indication for closure to prevent new vascular events.
Catheter closure is associated with a risk of procedure-related complications. Serious complications such as implant dislodgement, thrombus formation on the implant, pulmonary embolism and cardiac tamponade are rare, while minor bleeding and arrhythmias such as atrial fibrillation are more common (21, 22). To check for complications following the procedure, an echocardiogram is taken 1–2 months after discharge and again 6–12 months later.
Unusual presentations of known conditions can be clinically challenging. In patients who develop venous and arterial emboli, it is important to consider patent foramen ovale as a route for the emboli. Renal infarction is rare, with only a few cases described in the literature, but a missed or delayed diagnosis can lead to serious complications (3–7). In the young woman in the case report, we believe closure of the patent foramen ovale was the right course of action and crucial because the risk of new emboli causing a clinical stroke is assumed to be high.
The patient has consented to publication of this article.
The article has been peer-reviewed
- 1.
Naess IA, Christiansen SC, Romundstad P et al. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost 2007; 5: 692–9. [PubMed][CrossRef]
- 2.
Pastori D, Cormaci VM, Marucci S et al. A Comprehensive Review of Risk Factors for Venous Thromboembolism: From Epidemiology to Pathophysiology. Int J Mol Sci 2023; 24: 3169. [PubMed][CrossRef]
- 3.
Oh YK, Yang CW, Kim Y-L et al. Clinical Characteristics and Outcomes of Renal Infarction. Am J Kidney Dis 2016; 67: 243–50. [PubMed][CrossRef]
- 4.
Paris B, Bobrie G, Rossignol P et al. Blood pressure and renal outcomes in patients with kidney infarction and hypertension. J Hypertens 2006; 24: 1649–54. [PubMed][CrossRef]
- 5.
Hoxie H, Coggin C. Renal Infarction: statistical study of two hundred and five cases and detailed report of an unusual case. Arch Intern Med (Chic) 1940; 65: 587–94. [CrossRef]
- 6.
González-Bustos P, Roa-Chamorro R, Jaén-Águila F. Who shot first? Three possible causes of a kidney infarction. Clin Investig Arterioscler 2021; 33: 203–5. [PubMed][CrossRef]
- 7.
Saju JM, Leslie SW. Renal Infarction. Treasure Island, FL: StatPearls, 2023.
- 8.
Homma S, Messé SR, Rundek T et al. Patent foramen ovale. Nat Rev Dis Primers 2016; 2: 15086. [PubMed][CrossRef]
- 9.
Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc 1984; 59: 17–20. [PubMed][CrossRef]
- 10.
Zampieri F, Thiene G, Basso C et al. The three fetal shunts: A story of wrong eponyms. J Anat 2021; 238: 1028–35. [PubMed][CrossRef]
- 11.
Lippmann H, Rafferty T. Patent foramen ovale and paradoxical embolization: a historical perspective. Yale J Biol Med 1993; 66: 11–7. [PubMed]
- 12.
Jeong H, Woo Lee H, Young Joung J et al. Renal infarction caused by paradoxical embolism through a patent foramen ovale. Kidney Res Clin Pract 2012; 31: 196–9. [PubMed][CrossRef]
- 13.
Putaala J. Ischemic stroke in the young: Current perspectives on incidence, risk factors, and cardiovascular prognosis. Eur Stroke J 2016; 1: 28–40. [PubMed][CrossRef]
- 14.
Iizuka Y, Tsuchida T, Ashikaga K et al. Patent Foramen Ovale Complicated With Renal Infarction and Pulmonary Embolism: A Case Report With Literature Review. Cureus 2023; 15. doi: 10.7759/cureus.35433. [PubMed][CrossRef]
- 15.
Ho APT, Tjønnfjord EB, Schreiner C et al. A woman in her fifties with cirrhosis of the liver and postural dyspnoea. Tidsskr Nor Legeforen 2023; 143. doi: 10.4045/tidsskr.22.0754. [PubMed][CrossRef]
- 16.
Collado FMS, Poulin MF, Murphy JJ et al. Patent Foramen Ovale Closure for Stroke Prevention and Other Disorders. J Am Heart Assoc 2018; 7. doi: 10.1161/JAHA.117.007146. [PubMed][CrossRef]
- 17.
RESPECT Investigators. Long-Term Outcomes of Patent Foramen Ovale Closure or Medical Therapy after Stroke. N Engl J Med 2017; 377: 1022–32. [PubMed][CrossRef]
- 18.
CLOSE Investigators. Patent Foramen Ovale Closure or Anticoagulation vs. Antiplatelets after Stroke. N Engl J Med 2017; 377: 1011–21. [PubMed][CrossRef]
- 19.
Gore REDUCE Clinical Study Investigators. Patent Foramen Ovale Closure or Antiplatelet Therapy for Cryptogenic Stroke. N Engl J Med 2017; 377: 1033–42. [PubMed][CrossRef]
- 20.
Helsedirektoratet. Behandling av patent foramen ovale. https://www.helsedirektoratet.no/retningslinjer/hjerneslag/sekundaerforebygging-undersokelse-og-behandling-ved-hjerneslag/behandling-av-patent-foramen-ovale#patent-foramenovale-pfo-lukning-hos-pasienter-60-ar-med-hjerneinfarkt-uten-kjent-arsak-og-pavist-pfo Accessed 1.9.2023.
- 21.
Khairy P, O'Donnell CP, Landzberg MJ. Transcatheter closure versus medical therapy of patent foramen ovale and presumed paradoxical thromboemboli: a systematic review. Ann Intern Med 2003; 139: 753–60. [PubMed][CrossRef]
- 22.
Hirth A, Greve G, Rosland GA et al. [Transcatheter closure of patent foramen ovale in young stroke patients]. Tidsskr Nor Lægeforen 2003; 123: 785–8. [PubMed]