Main findings
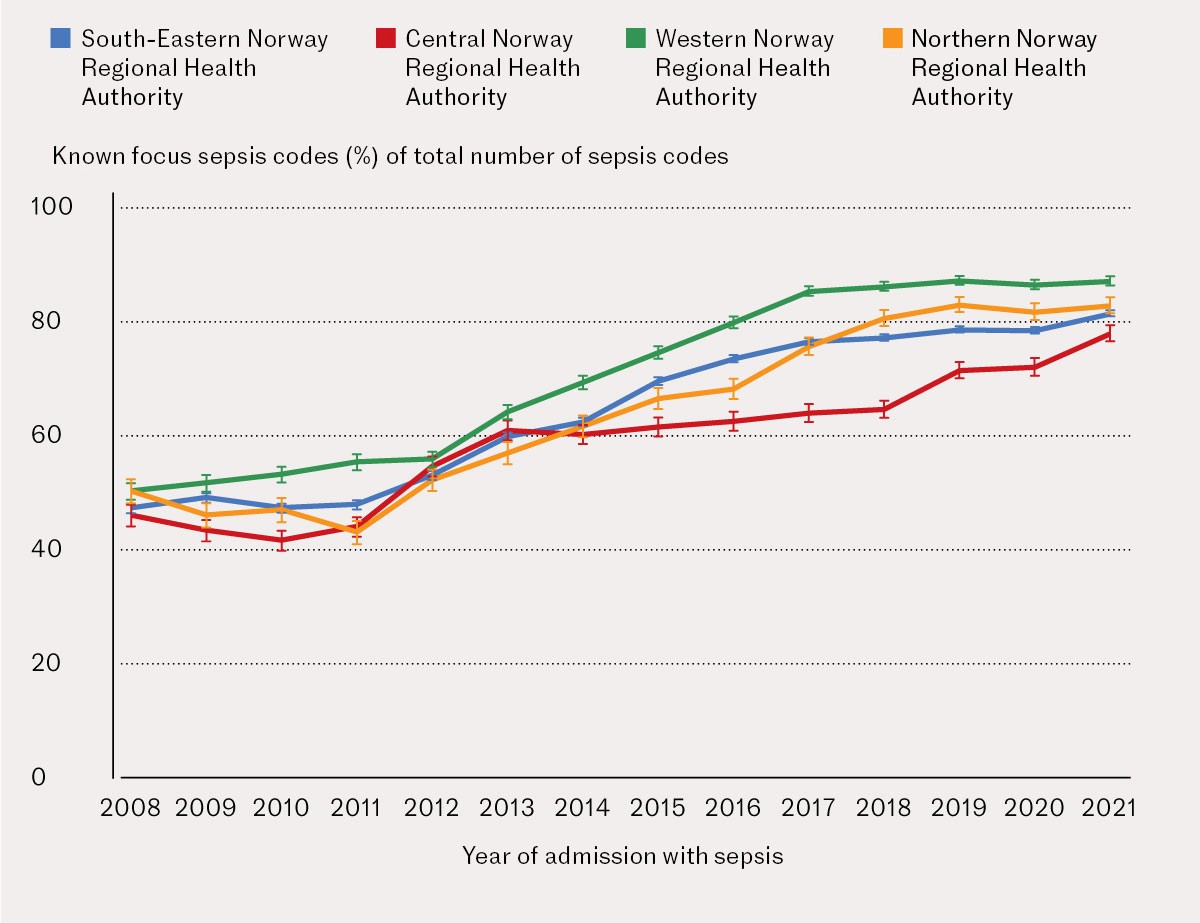
The greatest difference in coding practices among the regional health authorities in Norway was between the Western Norway Regional Health Authority and Central Norway Regional Health Authority, with an absolute difference of 12.9 % in the proportion of sepsis codes with a known focus between 1 January 2008 and 31 December 2021.
Medical codes identifying the focus of infection increased nationally by an average of 3.2 % per year, from 47.5 % in 2008 to 82.3 % in 2021.
Medical codes with an unknown focus decreased on a national basis by an average of 2.3 % per year, from 37.8 % in 2008 to 13.0 % in 2021.
Double registration with medical codes for known and unknown focus combined decreased nationally by an average of 0.9 % per year, from 14.3 % in 2008 to 4.1 % in 2021.
Introduction
Sepsis is defined today as an infection that triggers a dysregulated immune response, leading to life-threatening organ failure measured by a reduction in the Sequential Organ Failure Assessment (SOFA) score of at least two points (1). When a patient with sepsis is discharged from hospital, health authorities are required to report patients' diagnoses and procedure codes to the Norwegian Patient Registry. The purpose of medical coding is to ensure a clear understanding of conditions, symptoms and protocols across specialisations and organisations.
Reported codes are used for monitoring disease status, trend analyses and cause of death statistics. Medical codes also form the basis for activity-based funding in health authorities.
According to the national coding guidelines for ICD-10, sepsis should be coded as either 1) sepsis with an unknown focus of infection, or 2) sepsis with a known focus of infection, along with corresponding codes for the severity of acute organ failure, either code R65.1: Systemic inflammatory response syndrome (SIRS) of infectious origin, or code R57.2: Septic shock (2). Sepsis is particularly difficult to quantify and monitor because it can be caused by all types of infections and because the host response triggers acute organ failure in various organs. Sepsis therefore needs to be identified using a variety of medical codes and code combinations.
A review of sepsis coding practices in 2015 identified errors in the coding of A40 (streptococcal sepsis) and A41 (other sepsis) in up to 70 % of cases (3). It was determined that the diagnosis of sepsis was usually correct, but that the focus was recorded in the medical records in most cases and therefore should have been coded as sepsis with a known focus. Reducing the rate of coding errors can help increase confidence in patient data and further enhance the validity of research results. In addition, accurate and uniform coding practices provide a better basis for comparison between regions and thus better management by the regional health authorities. However, correct coding of sepsis is challenging, and since effective monitoring of this condition is crucial, there is a need to assess how sepsis is coded in Norway.
The objective of this national observational study was to examine sepsis coding practices in the period 1 January 2008 to 31 December 2021, with a particular emphasis on codes for known and unknown focus, as well as to compare code usage among Norway's four regional health authorities. In the years 2020 and 2021, sepsis also included COVID-19-related sepsis.
Material and method
Patient population
In this study, we used individual-based data from the Norwegian Patient Registry, including the date of each admission and discharge. The study included patients over the age of 17 with sepsis who were admitted to public hospitals in Norway in the period 1 January 2008–31 December 2021. Sepsis was identified and classified using ICD-10 codes based on the Sepsis-3 definition (1). We adapted the definition of sepsis to the national coding guidelines, which, unlike international literature, only includes focus of infection, not both agent and focus (2, 4, 5). Depending on whether the focus of infection was known or not, sepsis was divided into three categories: 1) known focus (code for the focus of infection in combination with a code for acute organ failure), 2) unknown focus (specific sepsis code), and 3) combination of both known and unknown focus (see the sepsis coding flowchart in the national coding guidelines) (Figure 1). Table 1 in the appendix shows all combinations of medical codes for the known and unknown focus categories.
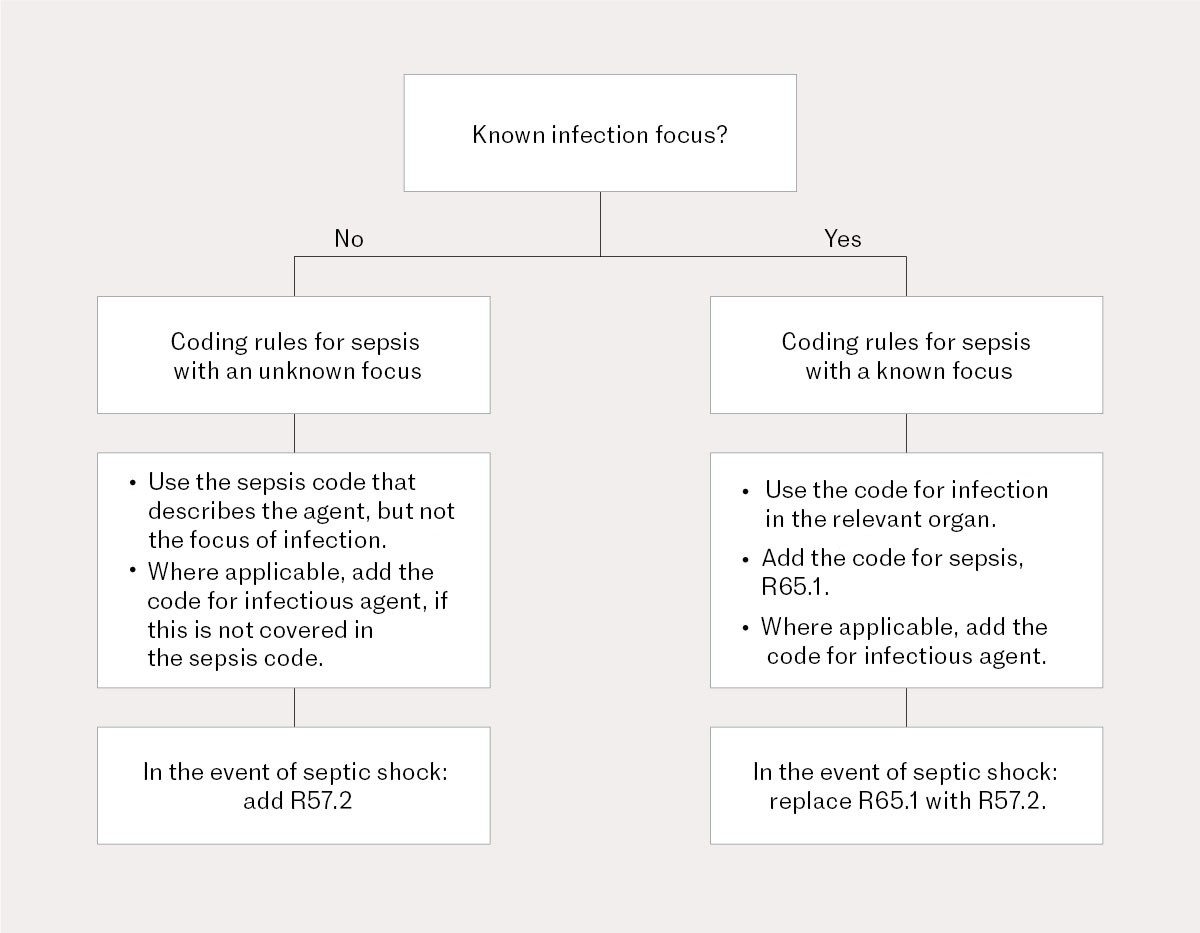
Table 1
Overview of the number and percentage of admissions at public hospitals in Norway for sepsis with a known and unknown focus, as well as with codes for both known and unknown focus in the period 1 January 2008–31 December 2021.
| Variables | Known focus1 | Unknown focus2 | Known and unknown focus3 | Total |
|---|---|---|---|---|
| Admissions4 | 210 391 (66.2) | 77 627 (24.4) | 29 687 (9.3) | 317 705 (100) |
| No. of acute organ failures | ||||
| 0 | 0 | 49 891 (64.3) | 25 929 (87.3) | 75 820 (23.9) |
| 1 | 173 965 (82.7) | 18 774 (24.2) | 3 732 (12.6) | 196 471 (61.8) |
| 2 | 28 554 (13.6) | 5 899 (7.6) | 24 (0.1) | 34 477 (10.9) |
| 3 | 5 889 (2.8) | 1 974 (2.5) | 2 (0.01) | 7 865 (2.5) |
| ≥ 4 | 1 983 (0.9) | 1 089 (1.4) | 0 | 3 072 (0.9) |
1Focus of infection is known
2Focus of infection is unknown, and includes all specific sepsis codes
3Code for known and unknown focus combined in the same admission
4Number of admissions for sepsis in the period 1 January 2008–31 December 2021
The known focus code requires the presence of concurrent acute organ failure. Acute organ failure was classified as a dichotomous variable per organ: organ failure 'Yes' (variable value 1) or 'No' (variable value 0). Any admission that involved sepsis codes was defined as a sepsis admission. An admission could consist of one or more codes for acute organ failure. Since sepsis with an unknown focus does not need to be coded together with organ failure, the number of organ failures was categorised from zero to four or more.
The codes defining COVID-19-related sepsis were incorporated into the study from 28 February 2020, the date of the first confirmed case in Norway. COVID-19-related sepsis (U07.1 or U07.2) was classified in the category 'known focus' if present with one or more codes for known focus, 'unknown focus' if present with unknown focus, and in the combination category 'known/unknown focus' if COVID-19 occurred together with both a code for unknown and known focus. Since there is no mandatory sequence of codes in the various diagnosis fields in the Norwegian Patient Registry, we searched for sepsis codes in one primary and up to 20 secondary diagnosis fields. (6).
The hospital regions were divided into four, according to the current health regions: South East, Central, West and North.
Statistical analysis
The Stata software package (version 16, Stata Corp, TX, USA) was used for all statistical analyses.
We estimated age-adjusted proportions as a percentage of the three coded categories of sepsis: 1) known focus (focus of infection plus acute organ failure), 2) unknown focus (specific sepsis code) and 3) combination of known/unknown focus. The age adjustment was performed using the 'dstdize' function in Stata, where all admissions in the population count as 1 to account for the use of individual-level data (7). We did this for the three aforementioned categories of sepsis per health authority and per year in the period 2008 to 2021. To identify trends in sepsis categories, we used least squares linear regression weighted by the inverse variance of the percentages with the specific ICD-10 codes, with 2008 as the reference year. This method allows calendar years with fewer sepsis cases to have less impact in the linear regression trend analysis over the years than calendar years with more sepsis cases.
Results are presented as frequencies, mean values or percentages, and unadjusted (raw) and age-adjusted percentages are reported with 95 % confidence intervals.
Ethics
The study was approved by the Regional Committee for Medical Research Ethics South East Norway (2019/42 772) and the Data Access Committee in Nord-Trøndelag Hospital Trust (2021/184). The analyses were performed at the Services for Sensitive Data, University of Oslo.
Results
Study population
During the study period, there were a total of 317 705 admissions for sepsis in 222 832 unique patients in Norwegian hospitals, distributed as follows: 210 391 (66.2 %) admissions for sepsis with a known focus, 77 627 (24.4 %) admissions with an unknown focus, and 29 687 (9.3 %) admissions with both a known and unknown focus. In total, there were 75 820 (23.9 %) sepsis admissions without acute organ failure and 196 471 (61.8 %) admissions with acute organ failure. A total of 34 477 (10.9 %), 7865 (2.5 %) and 3072 (0.9 %) admissions involved two, three, four or more acute organ failures respectively (Table 1).
1 Focus of infection is known
2 Focus of infection is unknown, and includes all specific sepsis codes
3 Code for known and unknown focus combined in the same admission
4 Number of admissions for sepsis in the period 1 January 2008–31 December 2021
During the period 28 February 2020 to 31 December 2021, there were a total of 3444 admissions with COVID-19-related sepsis. An overview of COVID-19 admissions is provided in Table 2 in the appendix.
Table 2
Distribution of known and unknown focus of infection, as well as combination of known/unknown focus in the four regional health authorities in the period 1 January 2008–31 December 2021. CI = confidence interval
| Known focus | Unknown focus | Known and unknown focus | |||||
|---|---|---|---|---|---|---|---|
| Health region | n | Raw % | Adjusted1 | Raw % | Adjusted1 | Raw % | Adjusted1 |
| South-East Norway | 179 286 | 65,6 | 65.7 (65.5– | 25.2 | 25.1 (24.9– | 8.7 | 8.6 (8.5–8.8) |
| Central Norway | 39 605 | 59,6 | 59.2 (58.7– | 27.8 | 27.7 (27.3– | 12.2 | 12.6 (12.3– |
| West Norway | 67 110 | 72,0 | 72.1 (71.8– | 20.6 | 20.5 (20.2– | 6.6 | 6.5 (6.3–6.7) |
| North Norway | 31 704 | 65,3 | 65.2 (64.7– | 24.4 | 24.4 (23.9– | 9.6 | 9.7 (9.4–10.0) |
1Adjusted for age
Adjusted for age, the highest percentage of sepsis codes with a known focus was in Western Norway Regional Health Authority, with 72.1 % (95 % CI: 71.8–72.5), while the lowest percentage was in Central Norway Regional Health Authority, with 59.2 % (95 % CI: 58.7–59.7), see Table 2.
Trends in sepsis coding
The changes in coding practices at national level follow an S-shaped curve, with the greatest changes occurring during the period 2012–17, then flattening out (Figure 2). The use of codes with a known focus increased on average by 3.2 % (95 % CI 2.7 to 3.6), from 47.5 % in 2008 to 82.3 % in 2021. The use of codes with an unknown focus decreased on average by 2.3 % (95 % CI -2.7 to
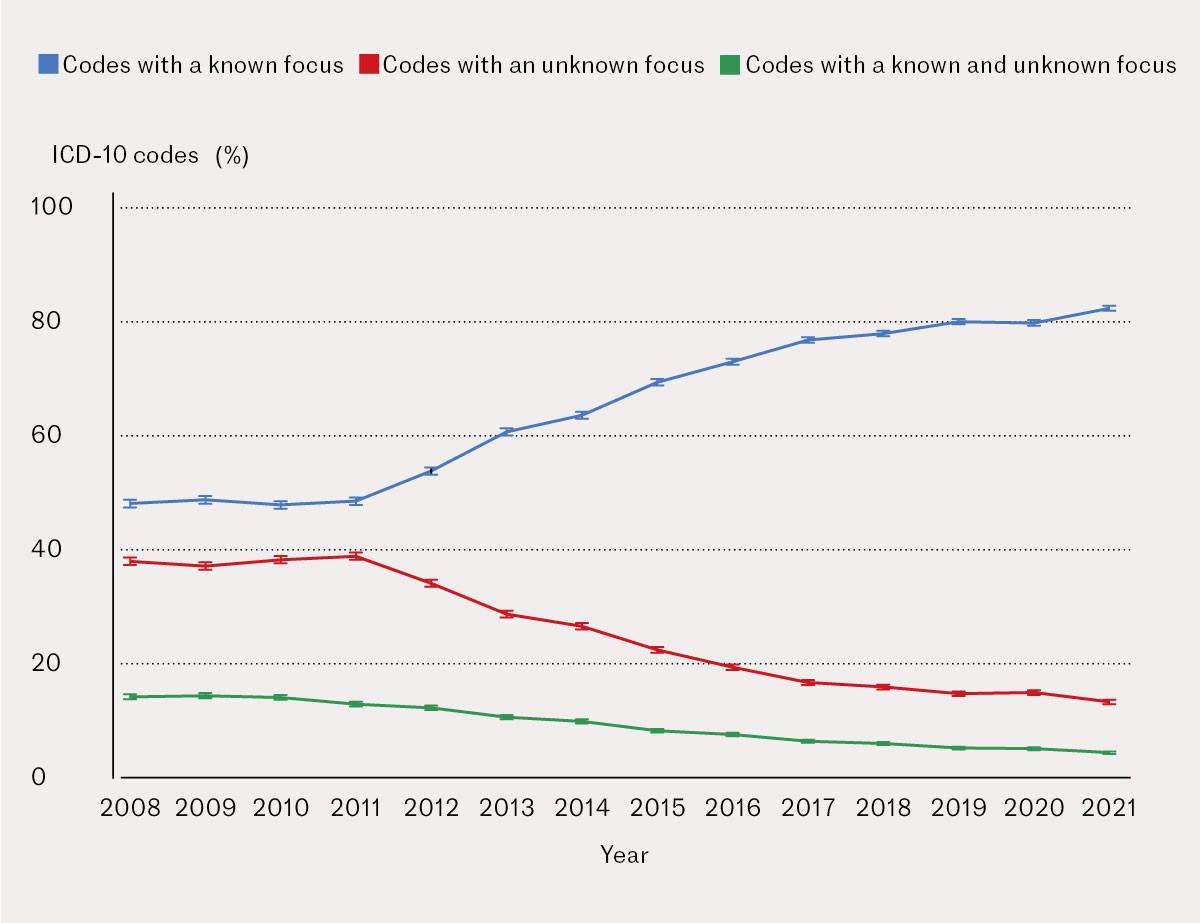
-1.9), from 37.8 % in 2008 to 13.0 % in 2021. The combination of known and unknown focus coded together decreased on average by 0.9 % (95 % CI -1.0 to -0.8) per year, from 14.3 % in 2008 to 4.1 % in 2021. Figure 3 shows a more detailed development of codes with a known focus for each of the four regional health authorities.
COVID-19-related sepsis was primarily coded with a known focus, but also occurred in conjunction with unknown focus and in the combination of known focus/unknown focus (see Table 2 in the appendix).
Discussion
In this observational study of sepsis spanning from 1 January 2008 to 31 December 2021, we found a significant variation in coding practices among the four regional health authorities, with a 12.9 % absolute difference in the proportion of sepsis codes with a known focus between Western Norway Regional Health Authority and Central Norway Regional Health Authority. The use of codes for known focus was also shown to have increased, while the use of codes with an unknown focus and double registration decreased during the study period.
To the best of our knowledge, the wide variation in the use of medical codes for sepsis between health authorities has not previously been shown in any study, indicating the need for a review of coding practices. Examples of an undesirable variation have, however, been observed previously: Kaspersen et al. found in 2018 that hospitals adhere to both national and local sepsis protocols, and only six hospitals used SOFA in their definition of sepsis (8). Coding practices between clinicians, departments and hospitals varied considerably, and access to clinical coders can vary across health regions (9). Previous studies have shown that clinical coders have been able to provide correct diagnostic codes in 90–95 % of cases, while clinicians have been shown to code correctly in 65–75 % of cases (9). To achieve the most consistent coding between hospitals and health regions, it is important to provide training in diagnostic coding for clinicians and to use clinical coders.
In 2018, Fleischmann-Struzek et al. showed that the use of sepsis codes for infection plus organ failure and unknown focus in Germany increased in the period 2007–13 (10). The opposite trend observed in our study in the use of codes with an unknown focus can be attributed to the more stable incidence of sepsis in Norway during the period 2008–21 compared to that reported in Germany (11, 12). In addition, the coding of acute organ failure is omitted from the coding guidelines' flowchart, leaving room for discretion in coding practices (2). We believe that including coding for acute organ failure in the coding guidelines' flowchart could make it easier for clinicians to adhere to the desired coding practice and help improve the quality of administrative data, thus enhancing the utility value of subsequent use of such data.
In Norwegian coding practices, coding is based on known and unknown focus with corresponding 'R' codes for sepsis, whereas in international literature, known focus is more broadly defined by including the agent of infection, in addition to focus plus organ failure (2, 4). The variation in coding practices complicates comparisons of epidemiological data between countries. It is hoped, however, that implementation of ICD-11, which is intended to facilitate a new classification of sepsis, will pave the way for more homogeneous coding across borders (13).
Admitting and treating patients with suspected sepsis and an unknown focus is a familiar problem for experienced clinicians, and the goal in such cases is to localise the infection. Codes for unknown focus, such as A40 (streptococcal sepsis), mean that the localisation of the focus is not known (2). Best practice should involve localising the infection and coding known focus where possible. Increasing the use of sepsis codes with a known focus will generate more detailed information about the sepsis diagnosis itself, which can be important for further follow-up and rehabilitation.
In this study, we found double registration of known and unknown focus in almost 10 % of admissions, with an annual reduction of 0.9 % between 2008 and 2021. The reduction in double registration and use of codes for unknown focus from 2012 can be explained by the new coding guidelines for sepsis, with the new codes for 'Systemic inflammatory response syndrome' and 'Septic shock', which were published in 2011. In 2021, over 80 % of all sepsis cases were coded with 'known focus', and this trend is contributing to more uniform coding and thus a higher quality of administrative data.
Our findings reveal major variations between the health regions in the coding of sepsis. These are believed to be multifactorial and may be due to unequal access to rapid microbiological diagnostics, geographical variations in primary care services, distance to hospitals, and variations in patient populations in the different health regions. Major variations in coding practices between health regions can lead to over- or under-funding, which indirectly creates geographical disparities in healthcare provision. Given the extent of the variation in our study, we recommend a new national review of sepsis coding.
A weakness of the study is that we used the Sepsis-3 definition as an extraction criterion from the Norwegian Patient Registry, despite this definition not coming into effect until 2016 (1). Sepsis is a heterogeneous syndrome, and in research and quality work in this field, the absence of a uniform method for identifying patients with sepsis is a challenge.
The definition of sepsis has changed over the years. From 1991 to 2015, sepsis was defined as infection plus deviation from at least two normal values measured by systemic inflammatory response syndrome (14, 15). The Sepsis-3 definition did not generate any new ICD-10 codes. However, the code for systemic inflammatory response syndrome after 2016 could be used when a patient's baseline in SOFA increased by at least two points. This change in coding practice may have compromised the comparability of data before and after the Sepsis-3 definition. There is also some uncertainty about the number of acute organ failures, since not all codes for organ failure need to be triggered by an infection, which may have led to an overestimation of sepsis codes with a known focus. The increased emphasis on sepsis in recent years through the Surviving Sepsis Campaign and the 2016–18 report by the Norwegian Board of Health Supervision may also have led to more cases of less severe infection being coded as sepsis, which in turn may have resulted in an overestimation (16, 17) . However, this mechanism, known as the Will Rogers phenomenon (18), is of less significance for this study since the incidence of all sepsis admissions in Norway was stable in the period 2008–21 (12). Furthermore, our dataset only includes information about the four regional health authorities, not the individual hospital trusts or hospitals, a decision made in consultation with the Regional Committees for Medical Research Ethics and the Norwegian Patient Registry for data protection purposes. It is also likely that disparities between health authorities are due to a combination of chance and the variation of patient populations, given the different role of university hospitals compared to local hospitals.
This study has numerous strengths, including our use of data from all public hospitals in Norway over a period of 14 years. Another strength lies in our adherence to the recommendation that a search for codes in a minimum of 15 diagnosis fields is needed to capture all relevant ICD-10 codes (6). Additionally, the validation of our medical codes for identifying sepsis and their use by other researchers further enhances the strength of our study (4, 5, 19). The fact that we adapted the codes to the Norwegian context in collaboration with a clinical research group (Mid-Norway Centre for Sepsis Research) and involved infectious disease specialists, microbiologists, anaesthesiologists and experts in medical coding ensured that the codes reflect actual clinical practice.
Conclusion
Our findings reveal variations in sepsis coding among the four regional health authorities, with a growing tendency for codes with a known focus and a reduction in instances of double registration and coding for unknown focus alone. It is important to continue the efforts aimed at ensuring more uniform coding, both for the planning of future healthcare services and for research and quality improvement work.
The article has been peer-reviewed.
- 1.
Singer M, Deutschman CS, Seymour CW et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315: 801–10. [PubMed][CrossRef]
- 2.
Direktoratet for e-helse. ICD-10 og ICD-11. https://www.ehelse.no/kodeverk-og-terminologi/ICD-10-og-ICD-11 Accessed 15.12.2022.
- 3.
Helsedirektoratet. Avregningutvalgets årsrapport 2015. https://www.helsedirektoratet.no/om-oss/rad-og-utvalg/avregningsutvalget/%C3%85rsrapport%20avregningsutvalget%202015.pdf/_/attachment/inline/390ae8bb-a542-4e99-8280-76d25d89b05d:eb7016da9e3ab493361c096ed887ed0bcd34cd55/%C3%85rsrapport%20avregningsutvalget%202015.pdf Accessed 19.1.2024.
- 4.
Angus DC, Linde-Zwirble WT, Lidicker J et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29: 1303–10. [PubMed][CrossRef]
- 5.
Rudd KE, Johnson SC, Agesa KM et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet 2020; 395: 200–11. [PubMed][CrossRef]
- 6.
World Health Organization Quality and Safety Topic Advisory Group. How many diagnosis fields are needed to capture safety events in administrative data? Findings and recommendations from the WHO ICD-11 Topic Advisory Group on Quality and Safety. Int J Qual Health Care 2014; 26: 16–25. [PubMed][CrossRef]
- 7.
STATA. dstdize — Direct and indirect standardization. https://www.stata.com/manuals13/rdstdize.pdf Accessed 19.1.2024.
- 8.
Kaspersen ER, Ræder J, Dahl V. Retningslinjer for behandling av sepsis. Tidsskr Nor Legeforen 2018; 138. doi: 10.4045/tidsskr.17.0493. [PubMed][CrossRef]
- 9.
Helse Sør-Øst. Medisinsk koding. https://www.helse-sorost.no/siteassets/documents/Kvalitet-og-pasientsikkerhet/Medisinsk-koding-v1.pdf Accessed 19.1.2024.
- 10.
Fleischmann-Struzek C, Thomas-Rüddel DO, Schettler A et al. Comparing the validity of different ICD coding abstraction strategies for sepsis case identification in German claims data. PLoS One 2018; 13. doi: 10.1371/journal.pone.0198847. [PubMed][CrossRef]
- 11.
Fleischmann C, Thomas-Rueddel DO, Hartmann M et al. Hospital Incidence and Mortality Rates of Sepsis. Dtsch Arztebl Int 2016; 113: 159–66. [PubMed]
- 12.
Skei NV, Nilsen TIL, Knoop ST et al. Long-term temporal trends in incidence rate and case fatality of sepsis and COVID-19-related sepsis in Norwegian hospitals, 2008-2021: a nationwide registry study. BMJ Open 2023; 13. doi: 10.1136/bmjopen-2023-071846. [PubMed][CrossRef]
- 13.
WHO. Sepsis. https://www.who.int/teams/integrated-health-services/infection-prevention-control/sepsis Accessed 7.3.2023.
- 14.
SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003; 31: 1250–6. [PubMed][CrossRef]
- 15.
The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 1992; 101: 1644–55. [PubMed][CrossRef]
- 16.
Surviving Sepsis Campaign. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 2010; 38: 367–74. [PubMed][CrossRef]
- 17.
Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41: 580–637. [PubMed][CrossRef]
- 18.
Iwashyna TJ, Angus DC. Declining case fatality rates for severe sepsis: good data bring good news with ambiguous implications. JAMA 2014; 311: 1295–7. [PubMed][CrossRef]
- 19.
Martin GS, Mannino DM, Eaton S et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003; 348: 1546–54. [PubMed][CrossRef]