Cytostatics can cause potentially fatal acute cardiovascular adverse effects.
A previously healthy woman in her forties with locally advanced rectal cancer was given radiotherapy (5 Gy x 5) before starting chemotherapy with a FOLFOX regimen (5-fluorouracil, oxaliplatin and folinic acid), as per the RAPIDO protocol (1). Curative surgery was also planned.
Approximately one and a half days after starting the first 5-fluorouracil infusion, she experienced burning pain in her chest and epigastrium, as well as nausea and vomiting, and she became generally unwell. Without interrupting the infusion, she was examined by a doctor at the oncology outpatient clinic the following morning, before being urgently admitted to the oncology ward for fluid, pain and nausea treatment.
Upon admission, she was in a reduced general condition and normotensive (blood pressure 123/74 mmHg), with a regular normal pulse rate (69 beats/min). Gastroscopy the following day showed minimal changes indicative of oesophagitis.
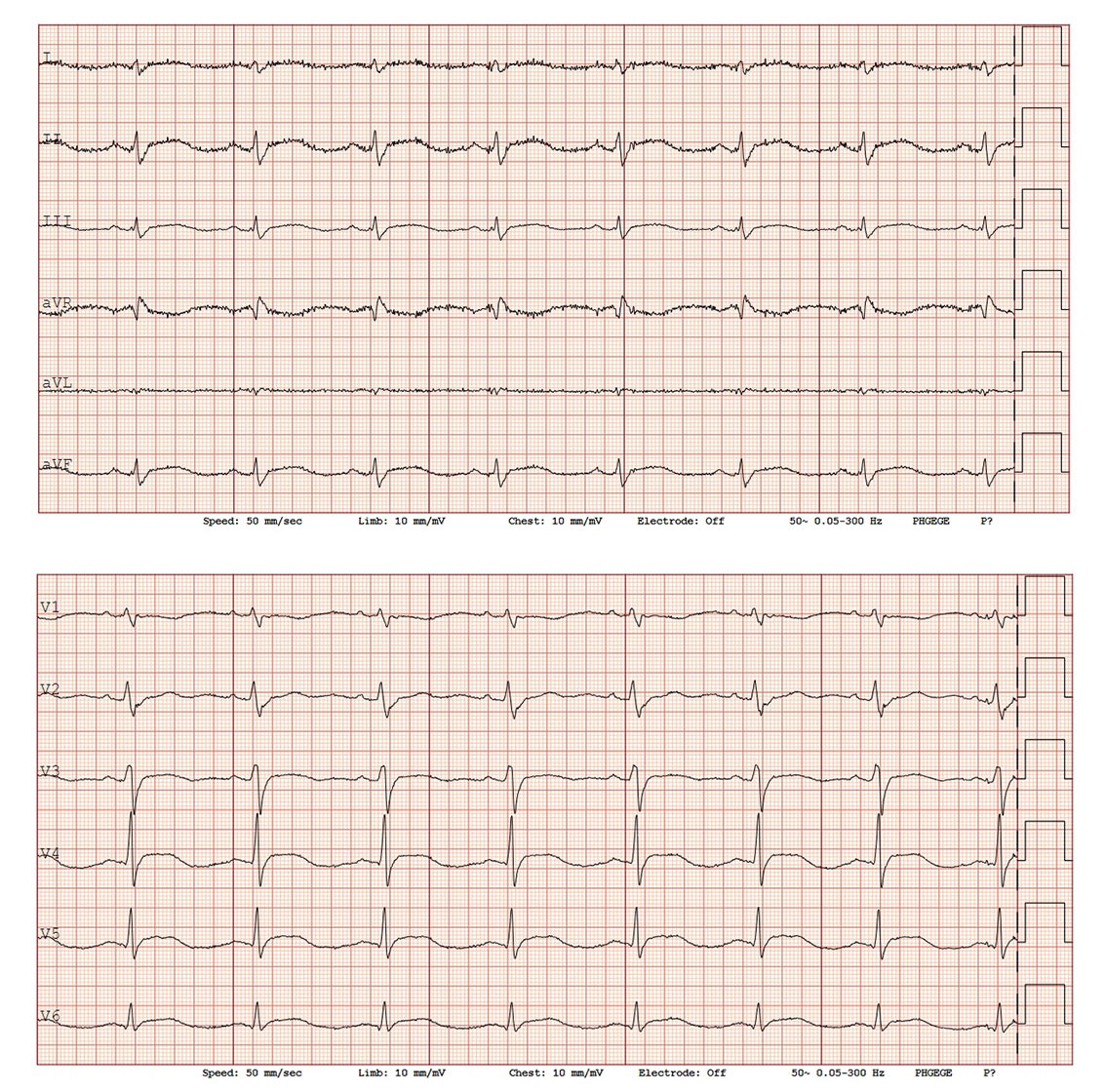
On the second day of hospitalisation, she was still nauseated and reported worsening chest pain, sometimes radiating to the shoulders and accompanied by numbness in the arms. The ECG showed mild ST elevation in leads II, III, aVF and V3–V6 (Figure 1). The corrected QT interval was normal, and there was no ECG for comparison. Blood tests revealed elevated troponin I of 239 ng/L (reference range 0–15 ng/L for women).
The patient was evaluated by a cardiologist. Echocardiography showed borderline dilation and eccentric hypertrophy of the left ventricle, hypokinetic segments from the middle section of the inferoposterior wall and lateral wall towards the apex, with moderately reduced left ventricular systolic function. Due to suboptimal apical imaging, the ejection fraction was uncertain.
A new ECG taken two hours after the first one showed newly developed T-wave inversions in V3–V6.
The overall clinical picture was interpreted as a possible inferior myocardial infarction, and the patient was transferred to the cardiology department. She was given 300 mg clopidogrel and 300 mg acetylsalicylic acid (aspirin) perorally for acute coronary syndrome, as well as 25 mg metoprolol extended-release tablets.
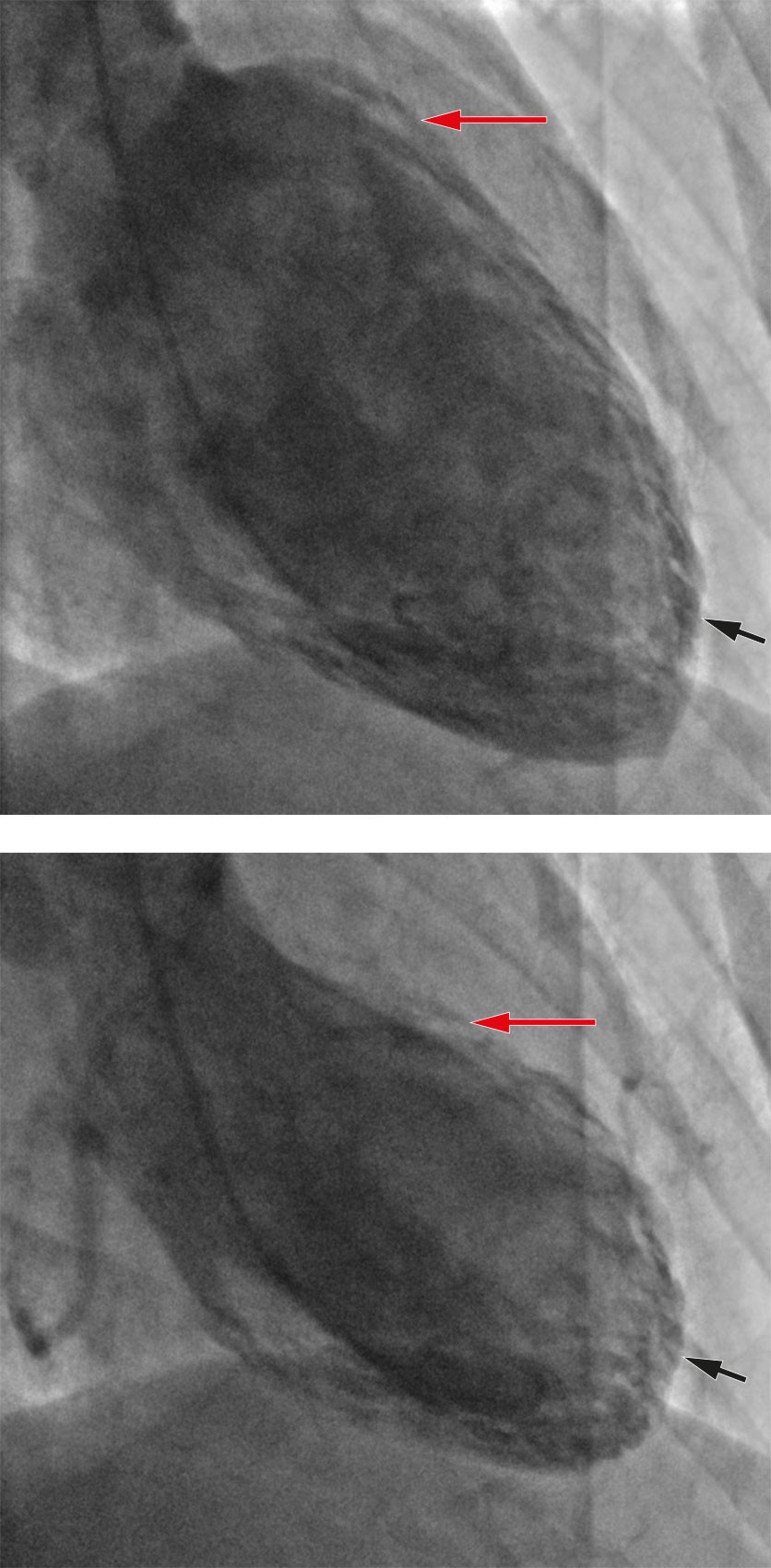
Coronary angiography revealed normal coronary arteries, and ventriculography showed apical hypokinesia and basal hypercontractility, typical for Takotsubo syndrome (Figure 2). Treatment with 1.25 mg ramipril was initiated that same evening.
During her hospital stay, the patient was haemodynamically stable with decreasing chest pain. Cardiac MRI with intravenous contrast on the fifth day of hospitalisation showed slightly reduced contractility in the left ventricle with apical hypokinesia and basal hyperkinesia, and an ejection fraction of 55 %. There were no signs of previous myocardial infarction or myocarditis. Platelet inhibitors and beta-blockers were therefore discontinued, but treatment with ramipril was continued.
Troponin I monitoring showed a decrease to 52 ng/L, and the patient was discharged five days after admission with suspected Takotsubo syndrome. Outpatient echocardiography three weeks later was normal.
Further treatment with 5-fluorouracil was discontinued due to suspicion of cardiotoxicity as a result of the treatment, and the case was reported as a suspected adverse effect. It was important to continue chemotherapy, and it was decided to replace 5-fluorouracil with tegafur/gimeracil/oteracil (Teysuno® tablets, S-1) in combination with oxaliplatin. The patient received three cycles with no signs of cardiotoxicity.
Discussion
Fluoropyrimidines such as 5-fluorouracil and capecitabine are common cytostatic agents used in gastrointestinal cancer and advanced breast cancer. Studies suggest that 3–4 % of patients have to stop treatment due to cardiotoxicity, typically presenting as angina pectoris, hypertension, myocardial infarction or Takotsubo syndrome (2, 3). Cardiotoxicity with 5-fluorouracil is most frequently observed during the first infusion, with a median of 12 hours from the start of infusion to symptom onset (4).
In 2021, the European Medicines Agency's safety committee decided to include Takotsubo syndrome as an adverse effect in the product information for 5-fluorouracil (5). The European Society of Cardiology's guidelines on cardiovascular disease investigation prior to chemotherapy recommend blood pressure measurement, ECG and measurement of lipid profile and HbA1c for all patients prior to starting fluoropyrimidines (3).
Takotsubo syndrome can present as acute coronary syndrome, but left ventricular dysfunction in Takotsubo syndrome is usually greater than the distribution area of a single coronary artery. Additionally, prolonged corrected QT interval and elevated NT-proBNP levels can increase the suspicion of Takotsubo syndrome. The condition predominantly affects women and is associated with physical and mental stress, including cancer and various cytostatic agents (2–3, 6–7).
An international group of experts has formulated the International Takotsubo (InterTAK) Diagnostic Criteria and proposed algorithms for investigating suspected Takotsubo syndrome (2, 6). For cancer patients, investigation according to standard algorithms is recommended (3).
Tegafur, which is included in Teysuno®, is a peroral fluoropyrimidine that metabolises to 5-fluorouracil in the liver (8). A large-scale retrospective study supports the claim that patients experiencing cardiotoxicity with 5-fluorouracil or capecitabine in an adjuvant or metastatic setting can switch to Teysuno® without a reduction in 5-year survival (9).
The patient has consented to the publication of this article.
The article has been peer-reviewed.
- 1.
Helsedirektoratet. Nasjonalt handlingsprogram med retningslinjer for diagnostikk, behandling og oppfølging av kreft i tykktarm og endetarm. https://www.helsedirektoratet.no/retningslinjer/kreft-i-tykktarm-og-endetarm-handlingsprogram Accessed 9.10.2023.
- 2.
Ghadri JR, Wittstein IS, Prasad A et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur Heart J 2018; 39: 2032–46. [PubMed][CrossRef]
- 3.
ESC Scientific Document Group. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J 2022; 43: 4229–361. [PubMed][CrossRef]
- 4.
Sara JD, Kaur J, Khodadadi R et al. 5-fluorouracil and cardiotoxicity: a review. Ther Adv Med Oncol 2018; 10. doi: 10.1177/1758835918780140. [PubMed][CrossRef]
- 5.
European Medicines Agency (EMA). Fluorouracil Accord (i.v. application). PSUSA-0000007-202012 – CMDh scientific conclusions and grouns for the variation to the terms of the Marketing Authorisation(s). https://www.ema.europa.eu/en/ Accessed 14.3.2023.
- 6.
Ghadri J-R, Wittstein IS, Prasad A et al. International expert consensus document on Takotsubo syndrome (part II): diagnostic workup, outcome, and management. Eur Heart J 2018; 39: 2047–62. [PubMed][CrossRef]
- 7.
Sattler K, El-Battrawy I, Lang S et al. Prevalence of cancer in Takotsubo cardiomyopathy: Short and long-term outcome. Int J Cardiol 2017; 238: 159–65. [PubMed][CrossRef]
- 8.
Statens legemiddelverk. Preparatomtale (SPC) Teysuno kapsler. https://www.legemiddelsok.no/ Accessed 9.10.2023.
- 9.
Osterlund P, Kinos S, Pfeiffer P et al. Continuation of fluoropyrimidine treatment with S-1 after cardiotoxicity on capecitabine- or 5-fluorouracil-based therapy in patients with solid tumours: a multicentre retrospective observational cohort study. ESMO Open 2022; 7. doi: 10.1016/j.esmoop.2022.100427. [PubMed][CrossRef]