A man in his seventies underwent routine heart examinations as part of workup for kidney transplantation. Unexpected findings led to more extensive investigations and revealed two rare systemic diseases as causes of his heart failure.
A man in his seventies was referred to a regional hospital for echocardiography and invasive coronary angiography as part of kidney transplant recipient workup. The patient had stage 5 chronic kidney disease and was receiving peritoneal dialysis. He did not have diabetes or hypertension. He had had stable monoclonal gammopathy of undetermined significance (MGUS) for 20 years. A renal biopsy taken ten years previously had revealed nephrosclerosis, but no deposition disease. Two years prior to the current admission, the patient had a cerebral stroke with sequelae in the form of dysarthria. Echocardiography at that time revealed mild left ventricular hypertrophy and diastolic dysfunction. He was receiving anticoagulant treatment with apixaban for permanent atrial fibrillation. He became generally fatigued on physical activity, but did not develop functional dyspnoea or chest pain.
During the elective admission to the regional hospital, the patient appeared to be in a good general condition without functional dyspnoea or chest pain. On clinical examination, there were normal findings on auscultation of the heart and lungs. He had no peripheral oedema. His blood pressure was 125/80 mmHg. Electrocardiography revealed atrial fibrillation with multifocal ventricular extrasystoles and mean ventricular heart rate of 70 bpm. QRS complex was 110 ms (normal value 80–120) with left axis deviation. Blood tests found elevated N-terminal pro-B-type natriuretic peptide (NT-proBNP) of 15,608 ng/L (0–500), erythrocyte sedimentation rate of 33 mm (1–12) and C-reactive protein of 7.1 mg/L (0–4). Creatinine was 589 µmol/L (60–105) and estimated glomerular filtration rate 7 mL/min/1.73 m2 (> 60), both in his usual range. Urea was 21.3 mmol/L (3.5–8.1) and potassium 4.3 mmol/L (3.6–4.6).
Echocardiography revealed global biventricular hypertrophy with end-diastolic septal thickness of 17 mm (6–10 mm) (Figure 1). There was decreased longitudinal shortening with relative apical sparing. The myocardium had a marked speckled appearance, particularly in the septum. The ejection fraction was 45 % (≥ 52) and the global longitudinal strain was −12 % (−18 to −22). The left atrium was dilated. No significant valve defects were discovered. The patient had no visible pericardial effusion. Coronary angiography was performed the following day and revealed no significant stenoses. Findings of increased wall thickness, speckled myocardium and marked decrease in longitudinal motion with apical sparing raised suspicion of deposition disease, and further investigation was initiated.
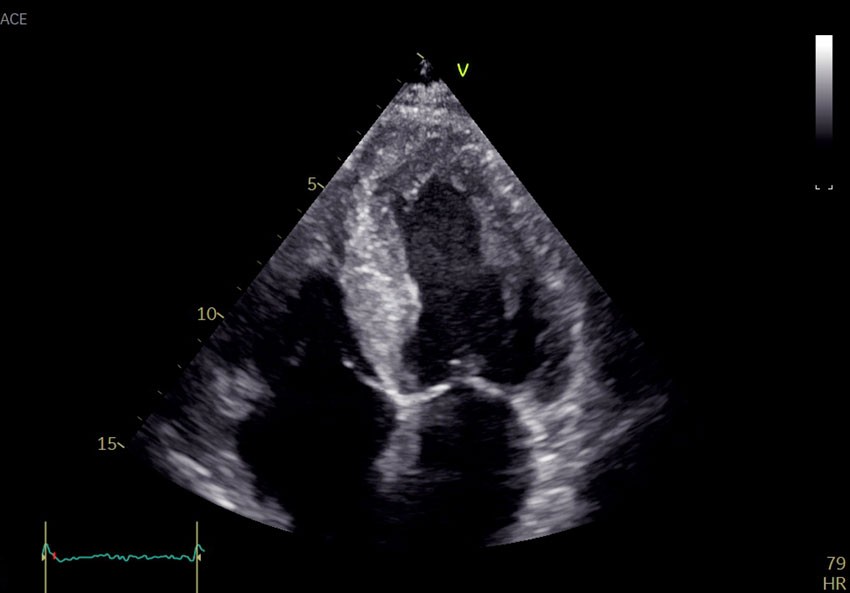
The most common cause of left ventricular hypertrophy is long-term hypertension, followed by hypertrophic cardiomyopathy (1). The latter is usually caused by sarcomeric disease, which is found to have a genetic cause in around half of cases. Deposition diseases such as amyloidosis and congenital metabolic disorder are rare differential diagnoses. Typical features of deposition diseases are biventricular hypertrophy and diastolic heart failure. In amyloidosis, the myocardium is seen to have a typical granular pattern on echocardiography and relatively preserved function in apical segments.
Amyloidosis is caused by precipitation and extracellular accumulation of protein components, amyloid fibrils. Amyloidosis is named after the protein that gives rise to the deposits. There are two types of systemic amyloidosis that most frequently affect the heart, transthyretin amyloidosis (ATTR amyloidosis) and immunoglobulin light chain amyloidosis (AL amyloidosis) (2). If cardiac amyloidosis is suspected, it is important to investigate both of these (pathogenetically unrelated) conditions. A few cases have been described in which the two types of systemic amyloidosis have occurred concurrently (3).
Based on the echocardiography findings, the attending doctor referred the patient for bone scintigraphy with technetium-labelled 3,3-diphosphono-1,2-propanodicarboxylic acid(99 mTc-DPD), which was performed five days later during the patient's hospital admission. Samples were also taken for urine and serum protein electrophoresis. Bone scintigraphy revealed high DPD uptake in the heart, Perugini grade 2 (Figure 2). Protein electrophoresis revealed monoclonal protein (M-protein) of IgA kappa type. There was elevation of free kappa chains (850 mg/L (4–25)) and free lambda chains (33 mg/L (6–27)). The kappa/lambda ratio was 25.8 (0.5–1.6), clearly abnormal.
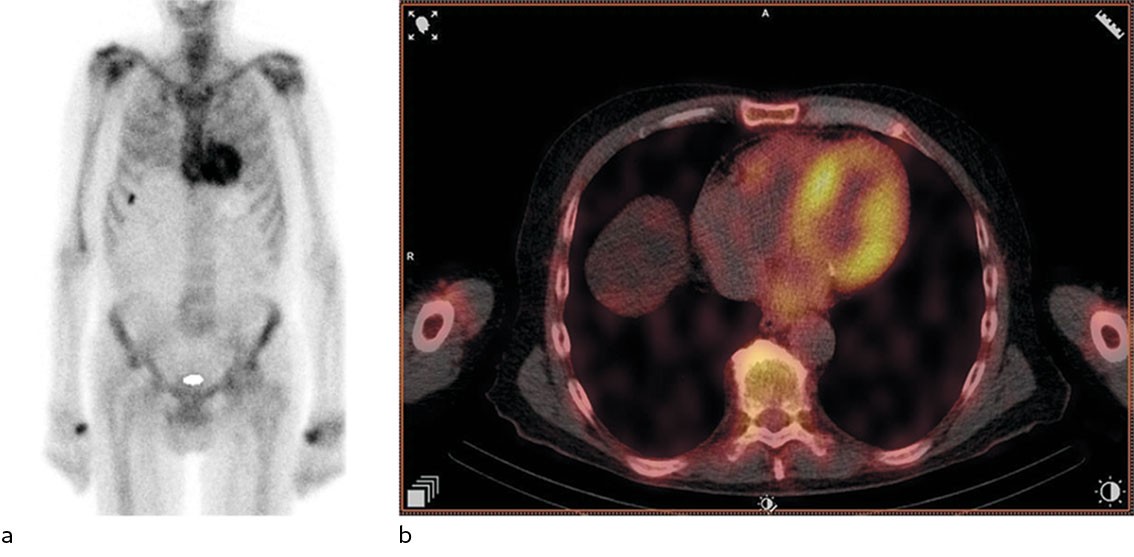
Bone scintigraphy is a nuclear medicine examination used when pathological conditions in the skeleton are suspected, for example metastases. 99 mTc-DPD scintigraphy has been shown to have very good diagnostic accuracy for cardiac ATTR amyloidosis, provided that AL amyloidosis has been ruled out by urine and serum protein electrophoresis (4). DPD uptake in the myocardium is graded on the Perugini scale from 0 to 3, based on myocardial uptake relative to skeletal uptake. Grades 2–3 are typically seen in ATTR amyloidosis (5). No uptake (grade 0) or slightly increased uptake (grade 1) are usually seen in AL amyloidosis, but grades 2–3 do not rule out AL amyloidosis (4). Several bisphosphonates, for example hydroxyl diphosphate and pyrophosphate, can also be used to detect cardiac amyloidosis.
Monoclonal gammopathy is diagnosed by findings of M-protein in electrophoresis of serum or urine (6). The M-protein consists of a specific immunoglobulin or one type of free light chain produced by one B-cell clone. If M-protein is detected, the patient undergoes further investigation to establish whether they have multiple myeloma, light or heavy chain disease or monoclonal gammopathy of undetermined significance (7). In AL amyloidosis, the disease is caused by deposition of light chains in the extracellular space (6). The diagnosis requires histological examination of tissue from the affected organ. Cardiac involvement is the main determinant of prognosis (8). In addition to cardiac involvement, there is often an effect on the kidneys, liver, gastrointestinal system and peripheral nerves (9). Concurrent multiple myeloma occurs in 10–15 % of patients with AL amyloidosis (7, 10).
The strong cardiac DPD uptake was consistent with ATTR amyloidosis. Since the patient had been found to have M-protein and an abnormal kappa/lambda ratio, AL amyloidosis had to be ruled out. A bone marrow biopsy was taken three weeks later at the patient's local hospital, and the patient was referred to another regional hospital for right heart catheterisation and cardiac biopsy. Right heart catheterisation was performed four weeks later and revealed mean pulmonary artery pressure of 22 mmHg (< 20) and slightly low cardiac output of 4.6 L/min (> 5). Biopsies from the septum were sent for histopathological examination.
Cardiac biopsy is performed by puncture of the internal jugular vein, and the biopsies are taken from the interventricular septum via the right ventricle. Cardiac biopsy has high diagnostic accuracy for amyloidosis because the deposits usually affect all areas of the myocardium. In amyloidosis, the myocardium is thickened and the risk of perforation is low. Right heart catheterisation is an invasive examination in which intracardiac pressure is measured using a fluid-filled end-hole catheter and cardiac output is measured using the thermodilution technique.
Histological examination of the endomyocardial biopsies revealed binding of Congo red, which is consistent with amyloid deposition. Immunostaining of the endomyocardial biopsies revealed the presence of both transthyretin and kappa light chains (Figure 3). Laser dissection with mass spectrometry revealed that both transthyretin and kappa light chains were among the main proteins, with transthyretin being most abundant, which is consistent with both cardiac transthyretin and amyloid light chain cardiomyopathy. Monoclonal plasma cells (kappa+) represented 10–12 % of the cells in the bone marrow biopsy. There were also amyloid deposits in the bone marrow. As part of the workup, fat pad biopsy was performed with no amyloid being found. Renal biopsy was not repeated. A CT scan found no lytic lesions in the skeleton.
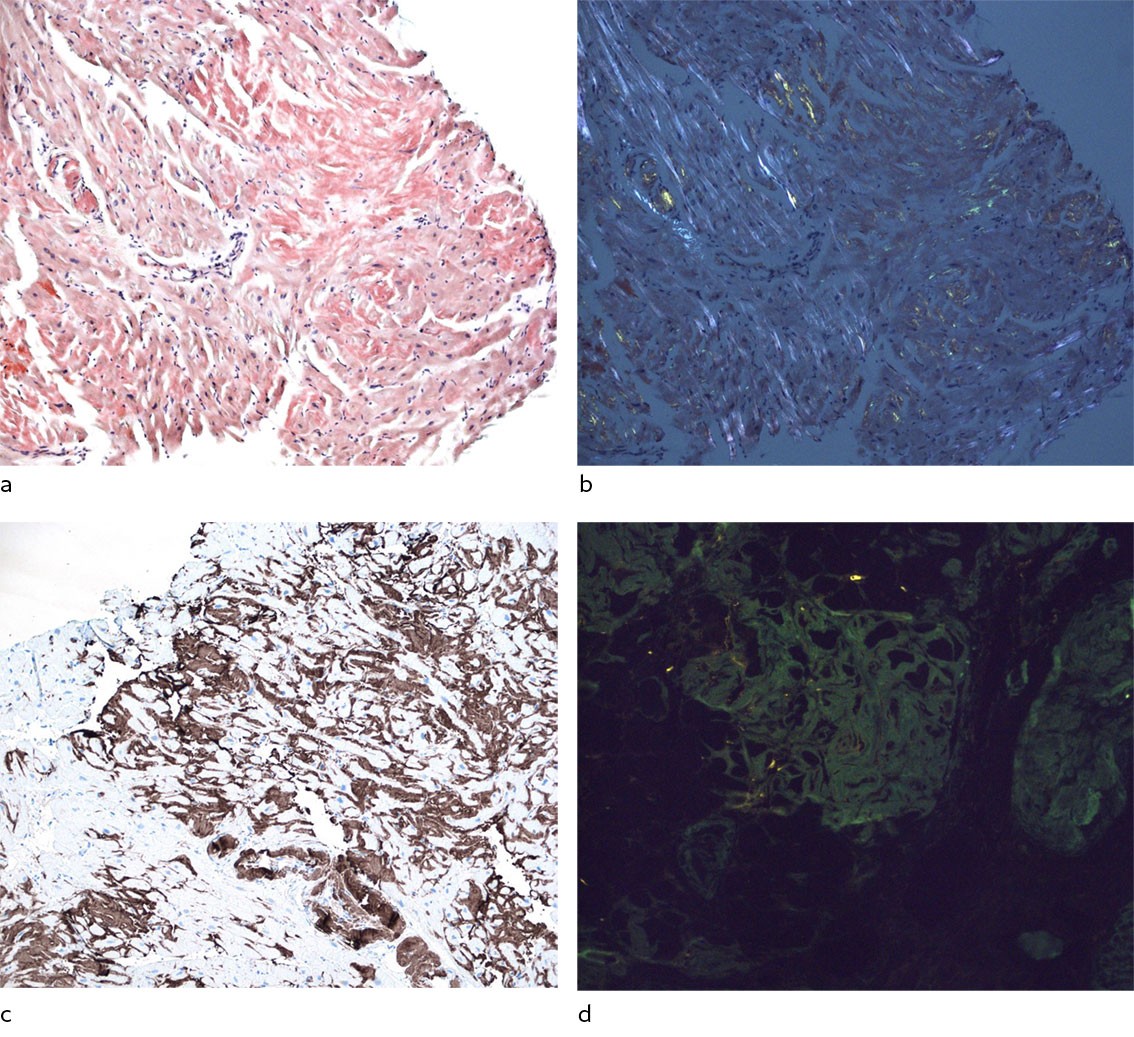
Amyloid consists of amorphous, extracellular deposits that displace normal tissue components and tissue function. Staining with Congo red is the gold standard for detection of amyloid, which appears orange-red and demonstrates apple-green birefringence under polarised light. There are 42 different proteins that have been identified as being able to form amyloid fibrils (11). Therefore, detection of amyloid should be followed by typing of the amyloidogenic protein. Immunohistochemistry has traditionally been used for typing (12). Laser dissection and subsequent mass spectrometry is a relatively new and advanced method with high sensitivity and specificity, making use of tissue that has already been provided for traditional histology. The method is available at Oslo University Hospital, and biopsies can be sent from all regional health authorities (13).
Challenging patient cases are discussed in a multidisciplinary working group for amyloidosis. The referring doctor is welcome to take part in the meeting in which the relevant case is to be discussed.
Our multidisciplinary group for amyloidosis discussed the clinical and radiological findings, blood test results and typing findings from the myocardial biopsy. The group has representatives from the fields of haematology, cardiology, nephrology, neurology, nuclear medicine, bioengineering, medical genetics, immunology, protein sequencing and pathology. We concluded that the patient probably had both ATTR and AL cardiac amyloidosis. The haematologist at the local hospital initiated treatment with melphalan, bortezomib and dexamethasone (Mel-Vel-Dex cycles) for AL amyloidosis. The patient had a haematological response to treatment, with normalisation of the kappa/lambda ratio.
The haematologist stopped treatment after five courses due to deterioration in the patient's general condition. Specific treatment of ATTR amyloidosis with tafamidis has no documented effect in patients with eGFR < 25 mL/min/1.73 m2 (14), and was therefore not initiated. The patient did not undergo kidney transplantation, but is now receiving haemodialysis three times a week. The patient is having regular follow-up with the haematologist at the local hospital. He is active and considers his general condition to be good.
Discussion
Cardiac amyloidosis is caused by deposition of amyloid fibrils in the extracellular space of the myocardium. Protein deposition causes the myocardium to become thick and stiffened, and leads to diastolic heart failure and arrhythmias. Both ATTR amyloidosis and AL amyloidosis are systemic diseases. Therefore, amyloid can be found in other organs besides the heart and can give rise to extracardiac symptoms/findings such as autonomic dysfunction, renal disease, spinal stenosis, carpal tunnel syndrome and peripheral neuropathy (9). In 2021, the European Society of Cardiology (ESC) Working Group on Myocardial and Pericardial Diseases published its recommendation for when cardiac amyloidosis should be suspected (Box 1) (15). The Working Group advocates for the importance of prompt diagnostic workup because targeted treatment is significant for patients' prognosis, particularly in AL amyloidosis.
Left ventricular hypertrophy ≥ 12 mm
And one or more of the following symptoms/findings:
Heart failure in patients ≥ 65 years
Aortic stenosis in patients ≥ 65 years
Hypotension or normotensive if previously hypertensive
Autonomic dysfunction
Peripheral polyneuropathy
Proteinuria
Increased bruising tendency
Bilateral carpal tunnel syndrome
Ruptured biceps tendon
Diffuse subendocardial/transmural gadolinium uptake or increased extracellular volume (CMR)
Reduced longitudinal strain with apical sparing (echo)
Decreased QRS voltage to mass ratio (ECG)
Pseudo Q waves (ECG)
AV conduction abnormalities (ECG)
Family history of amyloidosis disease
Cardiac phenotype and symptoms are the same in the two most common types of cardiac amyloidosis, ATTR amyloidosis and AL amyloidosis, but the conditions are treated differently and have different prognoses. ATTR amyloidosis is caused by deposits of misfolded transthyretin, a transport protein of retinol‐binding protein and thyroxine. The condition can be acquired or inherited. The typical patients with acquired ATTR amyloidosis are older men, but the condition also occurs in younger patients (16); we have diagnosed the condition in people in their fifties. Hereditary ATTR amyloidosis is related to point mutations in the gene that codes for transthyretin. Over 130 different point mutations have been described with variable penetrance and expressivity (17, 18). Hereditary amyloidosis is rare in people of Norwegian origin, but it is important to consider the possibility of hereditary ATTR amyloidosis, particularly in immigrants.
Symptomatic cardiac ATTR amyloidosis has a poor prognosis. In the Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR-ACT), approximately one-third of patients in the placebo group had died after two years. Tafamidis, a transthyretin stabiliser, is so far the only medicinal product that has been shown to improve survival in patients with ATTR amyloidosis (19). In Norway, the drug is available on an 'H prescription' (funded by regional health authorities) for patients who fulfil certain criteria. Drugs that suppress transthyretin transcription, such as vutrisiran, patisiran and inotersen, are being trialled (9, 15).
AL amyloidosis with cardiac involvement has an even worse prognosis than ATTR amyloidosis. Disease-specific treatment of AL amyloidosis involves reducing the production of the light chains, and thus the amyloid protein, by reducing the size of the B-cell clone. This is achieved with the use of both chemotherapy and immunotherapy regimens, as well as high-dose treatment with autologous stem cell rescue for selected patients (20).
Drug treatment of heart failure caused by amyloidosis differs from conventional heart failure treatment. Amyloidosis patients do not tolerate inhibition of the renin-angiotensin-aldosterone system well, due to the risk of hypotension. Beta-blockers must be used with caution due to a restrictive filling pattern, autonomic neuropathy and the risk of developing high-grade AV block (13, 21).
This case highlights several points. Amyloidosis is a possible underlying condition in patients with left ventricular hypertrophy. In cardiac amyloidosis, diagnostic evaluation based on imaging procedures and biochemistry alone is not always sufficient. Biopsy of the affected organ is key, particularly in suspected AL amyloidosis. This case illustrates the importance of multidisciplinary collaboration when investigating amyloidosis and other systemic diseases.
The patient has consented to the publication of the article.
The article has been peer-reviewed.
- 1.
Ommen SR, Mital S, Burke MA et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2020; 142: e558–631. [PubMed]
- 2.
Kittleson MM, Maurer MS, Ambardekar AV et al. Cardiac Amyloidosis: Evolving Diagnosis and Management: A Scientific Statement From the American Heart Association. Circulation 2020; 142: e7–22. [PubMed][CrossRef]
- 3.
Sidiqi MH, McPhail ED, Theis JD et al. Two types of amyloidosis presenting in a single patient: a case series. Blood Cancer J 2019; 9: 30. [PubMed][CrossRef]
- 4.
Gillmore JD, Maurer MS, Falk RH et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016; 133: 2404–12. [PubMed][CrossRef]
- 5.
Perugini E, Guidalotti PL, Salvi F et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol 2005; 46: 1076–84. [PubMed][CrossRef]
- 6.
Tjønnfjord GE, Schjesvold FH, Gulbrandsen N et al. Monoklonal gammopati av klinisk betydning. Tidsskr Nor Legeforen 2021; 141. doi: 10.4045/tidsskr.20.0981. [PubMed][CrossRef]
- 7.
Schjesvold FH. En innføring i lettkjede-amyloidose. Indremedisineren 19.6.2013. https://indremedisineren.no/2013/06/en-innforing-i-lettkjede-amyloidose/ Accessed 23.4.2023.
- 8.
Gertz MA. Immunoglobulin light chain amyloidosis: 2020 update on diagnosis, prognosis, and treatment. Am J Hematol 2020; 95: 848–60. [PubMed][CrossRef]
- 9.
Gertz MA, Dispenzieri A. Systemic Amyloidosis Recognition, Prognosis, and Therapy: A Systematic Review. JAMA 2020; 324: 79–89. [PubMed][CrossRef]
- 10.
Rajkumar SV, Kyle RA, Therneau TM et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood 2005; 106: 812–7. [PubMed][CrossRef]
- 11.
Buxbaum JN, Dispenzieri A, Eisenberg DS et al. Amyloid nomenclature 2022: update, novel proteins, and recommendations by the International Society of Amyloidosis (ISA) Nomenclature Committee. Amyloid 2022; 29: 213–9. [PubMed][CrossRef]
- 12.
Vrana JA, Gamez JD, Madden BJ et al. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood 2009; 114: 4957–9. [PubMed][CrossRef]
- 13.
Wien TN, Kvam AK, Stensland M et al. Veileder for diagnostikk og behandling av amyloidose. https://www.helsebiblioteket.no/innhold/retningslinjer/veileder-for-diagnostikk-og-behandling-av-amyloidose Accessed 23.4.2023.
- 14.
Heidenreich PA, Bozkurt B, Aguilar D et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022; 145: e876–94. [PubMed][CrossRef]
- 15.
Garcia-Pavia P, Rapezzi C, Adler Y et al. Diagnosis and treatment of cardiac amyloidosis. A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur J Heart Fail 2021; 23: 512–26. [PubMed][CrossRef]
- 16.
Lane T, Fontana M, Martinez-Naharro A et al. Natural History, Quality of Life, and Outcome in Cardiac Transthyretin Amyloidosis. Circulation 2019; 140: 16–26. [PubMed][CrossRef]
- 17.
Damy T, Kristen AV, Suhr OB et al. Transthyretin cardiac amyloidosis in continental Western Europe: an insight through the Transthyretin Amyloidosis Outcomes Survey (THAOS). Eur Heart J 2019; 43: 391–400. [PubMed][CrossRef]
- 18.
Suhr OB, Svendsen IH, Andersson R et al. Hereditary transthyretin amyloidosis from a Scandinavian perspective. J Intern Med 2003; 254: 225–35. [PubMed][CrossRef]
- 19.
Maurer MS, Schwartz JH, Gundapaneni B et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med 2018; 379: 1007–16. [PubMed][CrossRef]
- 20.
Schjesvold FH. Al-amyloidose i hjertet – vi finner pasientene for sent! Hjerteforum 2017; 30: 13–9.
- 21.
Selvanayagam JB, Hawkins PN, Paul B et al. Evaluation and management of the cardiac amyloidosis. J Am Coll Cardiol 2007; 50: 2101–10. [PubMed][CrossRef]