Perinatal asphyxia is caused by a number of conditions that occur during the perinatal period and which lead to hypoxia, ischaemia, hypercapnia and metabolic acidosis (1). Symptoms and findings that indicate perinatal asphyxia include hypoxic ischaemic encephalopathy (HIE) with (sub-)clinical seizures and reduced level of consciousness. Some causes of perinatal asphyxia are shown in Table 1. Perinatal asphyxia occurs in 1 – 10 of 1 000 births, with a different incidence in different parts of the world. The condition causes more than 800 000 deaths in the neonatal period per year worldwide and a substantial proportion of the children that survive suffer late effects such as cerebral palsy and epilepsy (3).
Table 1
Some causes of perinatal asphyxia, grouped into conditions before (antepartum), during (intrapartum) and after birth (postpartum) (2)
| Antepartum |
Intrapartum |
Postpartum |
| Umbilical cord compression |
Umbilical cord compression |
Respiratory depression due to opiates in mother's circulatory system |
| Anaemia |
Anaemia |
Obstructed airway |
| Bleeding |
Bleeding |
Congenital sepsis |
| Uterine hyperactivity |
Uterine hyperactivity (for example as a result of over-stimulation) |
Congenital heart defect and / or lung anomalies |
| Placental abruption |
Placental abruption |
|
| Placental dysfunction |
Uterine rupture |
|
| Birth dystocia |
||
| Traumatic delivery/birth trauma |
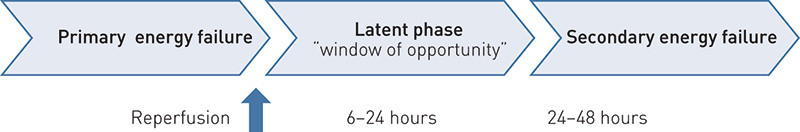
The mechanisms that cause neurological damage after perinatal asphyxia are divided schematically into three metabolic phases (4) (Fig. 1) (5, 6), and the objective of the treatment is to limit ongoing damage to cells. Hypoxia leads to primary energy failure (phase 1), but a short time after reoxygenation, aerobic metabolism and cell functions are re-established (phase 2). However, as a result of a cascade of cellular mechanisms (7, 8), after this «latent phase» of 6 – 24 hours mitochondrial energy production again begins to fail. This secondary energy failure (phase 3) lasts for 24 – 48 hours after the hypoxic event. The damage that occurs during this phase is considerable (9). Neuroprotective treatment targeting the «latent phase» may limit the secondary neuron damage due to perinatal asphyxia (5) (Fig. 1). New knowledge about cellular repair mechanisms can also pave the way for types of treatment that not merely limit damage, but can also repair defects in the immature nervous system (10).
The purpose of this article is to provide an overview of established measures to limit neurological damage after perinatal asphyxia. Since these are limited in number, a selection of proposed strategies that appear promising for the future treatment of perinatal asphyxia is also presented.
Method
We searched in the Medline and Cochrane Library databases on the search terms «perinatal asphyxia», «asphyxia neonatorum», «hypoxic ischemic encephalopathy» OR «hypoxia-ischemia, brain»; «treatment», «therapeutics» OR «neuroprotective agents» AND «infant, newborn». No restrictions were placed on the age of the publications. Only publications in English were included. The search was concluded on 9 December 2011. An overwhelming number of potential treatments were identified. On the basis of discussions with leading specialists in neonatal research, published abstracts from international paediatrics congresses in 2011 and publications from our own research group, we are presenting the interventions that appear most promising following a discretionary appraisal. We have also included more innovative methods based on research at our own institute, such as cell-based treatment and DNA repair mechanisms. These methods are discussed in 44 Medline-indexed articles and a Cochrane overview from our literature search. The remaining references in this article are about asphyxia and mechanisms generally and do not deal with neuroprotection in particular.
Results
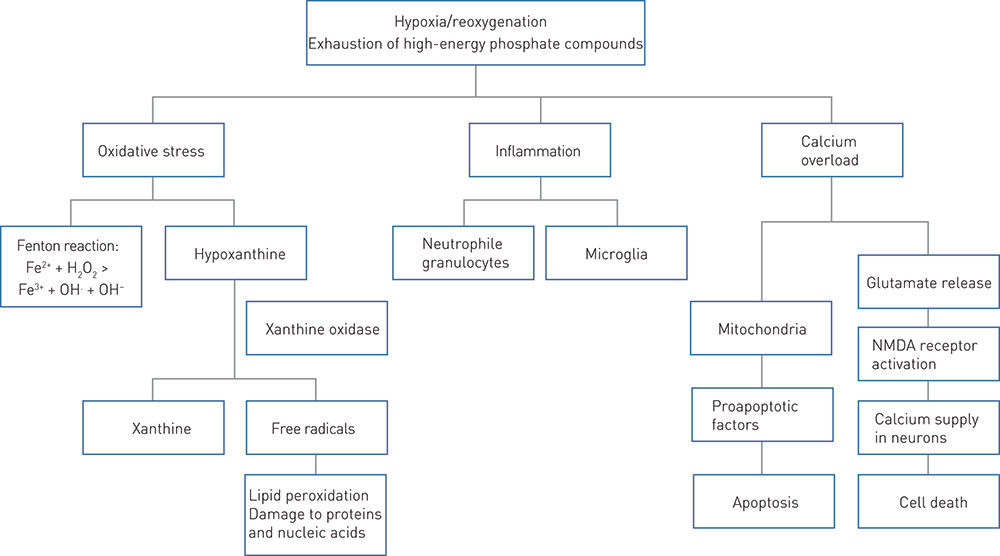
Mechanisms that lead to cell damage after perinatal asphyxia are presented schematically in Fig. 2 (11). In the following, neuroprotective strategies are grouped according to where in this chain they act. For the sake of simplicity, each strategy is presented under a single heading, even though many treatments have more than one mechanism. The strategies are also classified as non-medicinal, medicinal or cell-based.
Non-medicinal treatment
General. Prevention through good perinatal care and appropriate treatment and stabilisation immediately after birth are important. Experimental and clinical studies have shown that hyperoxia after perinatal asphyxia exacerbates damage in both animals and humans (12, 13). Since the use of extra oxygen in resuscitation after asphyxia does not improve the short-term outcome clinically or experimentally either (14, 15), hyperoxia should be avoided and moderate hypoxaemia should possibly even be sought (16).
Hypothermia. Therapeutic hypothermia for moderate to severe perinatal asphyxia has become established treatment (17). Studies have shown that hypothermia reduces cellular energy utilisation (18), anaerobic metabolism and the formation of free radicals (19). Hypothermia also reduces the release of glutamate (19) and proapoptotic factors (6). In Norway, hypothermia treatment of newborns is a centralised task, and is performed according to a standardised protocol from the large, randomised clinical hypothermia trials (20) – (22). All maternity units are to have routines for transfer to regional cooling centres of newborns with a gestation age ≥ 36 weeks with moderate to severe perinatal asphyxia, even though randomised clinical trials have shown hypothermia to be the most effective for moderate asphyxia (23). Cooling must be initiated within six hours of the hypoxic event (in practice most often from the time of birth) and may take the form of whole body hypothermia or selective cooling of the head. With whole body hypothermia as practised in Norway, moderate hypothermia is sought, defined as rectal temperature of 33.5°C for 72 hours before gradual re-warming.
Medicinal treatment
Antioxidant effect. Allopurinol is used to treat gout and inhibits hypoxanthine catabolism, which generates free radicals after hypoxia/ischaemia (24) (Fig. 2). Allopurinol also acts by directly neutralising free radicals and binds free iron which is released from proteins by hypoxia (25). Free iron reacts with hydrogen peroxide and forms toxic hydroxyl radicals (11).
A Cochrane review from 2010 (26) included three randomised clinical trials (24, 27, 28). In these trials, allopurinol administered intravenously within two and four hours of birth in a total dose of 40 mg/kg did not result in a reduction in mortality, seizure frequency or number of pathological findings in cerebral imaging in the neonatal period. However, allopurinol treatment reduced the incidence of the combined outcome of death and severe handicap at the age of four to eight years when the children with the most severe asphyxia were excluded from the analysis (29). A clinical trial of antenatal allopurinol is in progress (30).
N-acetyl cysteine is used as a mucous-clearing treatment and for paracetamol overdose. N-acetyl cysteine is claimed to traverse the placenta and blood-brain barrier (31), can be safely used during pregnancy (32) and is a source of L-cysteine which is necessary for the formation of the endogenous antioxidant glutathione (31). Studies of neonatal pigs have shown that N-acetyl cysteine administered as an intravenous bolus of 150 mg/kg or 30 mg/kg ten or five minutes after the start of reoxygenation, followed by 100 mg/kg/hour or 20 mg/kg/hour reduces oxidative stress after hypoxia/ischaemia and improves systemic and cerebral haemodynamics (33, 34).
Glutamate antagonists. Xenon is a glutamate N-methyl-D-aspartate receptor antagonist (NMDA receptor antagonist) and has neuroprotective effects both in vitro (35) and in vivo (7). Inhalation of 5011% xenon for 18 hours combined with therapeutic hypothermia is undergoing clinical testing (Marianne Thoresen, unpublished lecture, Pediaterdagene [Paediatric days] 2012).
In addition to neuroprotection, xenon stabilises cardiovascular functions (36) and protects the myocardium (37).
One major disadvantage of xenon is that the gas is rare and expensive. Researchers who study xenon inhalation in newborns have therefore developed systems for recycling the gas in a closed system (38).
Anti-inflammatory treatment. Erythropoietin (EPO) is a haematopoietic hormone that also has receptors elsewhere than in haematopoietic tissue, including the brain (39).
As well as having an anti-inflammatory effect, EPO can reduce brain damage after hypoxia through reduced nitric oxide (NO) production (40), inhibited glutamate toxicity (41) and reduced lipid peroxidation (42). Neuronal anti-apoptotic mechanisms, angiogenesis and neurogenesis are also stimulated and modulated (43).
300 units/kg or 500 units/kg recombinant EPO administered subcutaneously within 48 hours of birth have been shown to improve the neurological outcome after perinatal asphyxia, with the most pronounced effect on girls (44). Higher doses (2 500 units/kg) given within 4 – 6 hours to newborns with mild/moderate hypoxic ischaemic encephalopathy have also had a positive effect on seizure control, EEG background activity and neurological development at the age of six months (45). Trials are now being conducted on EPO combined with hypothermia treatment.
Cell-based treatment
Stem cell treatment. Stem cell treatment can be administered by stimulating endogenous stem cells or by transplanting exogenous stem cells. There is a theoretical rationale supported by experimental animal studies to the effect that the administration or stimulation of neurotrophic factors/growth factors such as EPO, insulin-like growth factors and brain-derived neurotrophic factors can reduce brain damage after perinatal asphyxia (46) – (49). Mesenchymal stem cells from umbilical cord blood are possibly the most promising option for exogenous stem cell treatment of brain damage after perinatal asphyxia (50).
DNA repair enzymes. Hypoxia leads to damage to DNA bases. Endogenous repair mechanisms involve DNA repair enzymes including DNA glycosylases. Sejersted et al. recently published a study of the DNA glycosylase Neil-3 (endonuclease VIII-like 3) (10). In this study, Neil-3 knockout mice had a reduced ability to regenerate damaged brain tissue, probably as a result of both reduced proliferation of neuronal progenitors and inhibition of the ability to differentiate into mature neurons. The knowledge yielded by these findings provides a basis for treatment targeting endogenous repair mechanisms.
Discussion
Hypothermia is currently the only neuroprotective treatment with a documented efficacy and safety profile in randomised clinical trials (20) – (22). However, the number needed to treat in order for one to survive without moderate to severe sequelae is 8 – 9, and almost half of all children treated with hypothermia suffer permanent neurological damage (23). There is therefore a need to optimise this form of treatment, for example by starting cooling earlier, changing the depth (temperature) of cooling and/or giving supplements of potentiating drugs.
Preclinical and clinical research now focuses on the additive or synergistic effects of hypothermia in combination with other neuroprotective treatment options (51, 52). Hypothermia changes the mechanisms of injury after perinatal asphyxia, as well as the pharmacokinetics and pharmacodynamics of drugs. It is therefore necessary to make a thorough study of the optimal time window, doses and any side effects both in vitro and in animals before clinical trials take place.
A combination of treatment methods targeting different mechanisms of injury may be necessary for achieving the best possible outcome after perinatal asphyxia. Today xenon, EPO and allopurinol treatment are the most convincing, and randomised clinical trials are on the way. Cell-based treatment methods and treatment targeting antioxidant defence also appear promising and may be important in future treatment of infants affected by asphyxia.
The article is based on a trial lecture by a doctoral candidate at the Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, 9 December 2011.
MAIN POINTS
Measures to limit neurological damage after perinatal asphyxia focus on the «latent phase» 6 – 24 hours after the time when the damage occurred
Therapeutic hypothermia is currently the only established treatment for perinatal asphyxia
Xenon gas, allopurinol and erythropoietin may possibly reinforce the neuroprotective effect of therapeutic hypothermia
- 1.
Rennie JM. Roberton's textbook of neonatology. London: Churchill Livingstone, 2005: 1128.
- 2.
McGuire W. Perinatal asphyxia. Clin Evid 2006; nr. 15: 511 – 9. [PubMed]
- 11.
Bolann BJ, Ulvik RJ. Release of iron from ferritin by xanthine oxidase. Role of the superoxide radical. Biochem J 1987; 243: 55 – 9. [PubMed]
- 21.
Gluckman PD, Wyatt JS, Azzopardi D et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005; 365: 663 – 70. [PubMed]
- 26.
Chaudhari T, McGuire W. Allopurinol for preventing mortality and morbidity in newborn infants with suspected hypoxic-ischaemic encephalopathy. Cochrane Database Syst Rev 2008; nr. 2: CD006817. [PubMed]