In the 1950s and 60s, the classic psychedelic drugs lysergic acid diethylamide (LSD) and psilocybin were tested in numerous clinical trials. The results suggested efficacy against anxiety and depression in cases of life-threatening illness, unipolar depression and addiction (1–3). An open study of LSD in cancer patients demonstrated improvement in symptoms such as pain, anxiety and depression and a greater acceptance of death in approximately 70 % of the sample (1). A review of open studies examining the efficacy of psychedelic drugs in unipolar depression showed an improvement in approximately 80 % of patients (2). A meta-analysis of randomised studies of LSD in cases of alcohol misuse demonstrated significant efficacy of LSD relative to the control group, with an odds ratio of 1.96 (3). The use of psychedelic drugs in treatment and research ended around 1970 as a result of international legislation (4). Psychedelic drugs are classified by the United Nations as substances with potential for abuse, with serious adverse effects on public health and no therapeutic potential, but this classification has been criticised (5).
Classic psychedelic drugs may be synthetic (such as LSD) or naturally occurring, such as psilocybin from psilocybin ('magic') mushrooms, N,N-dimethyltryptamine (DMT) from the herbal drink ayahuasca, and mescaline from the Peyote cactus. All are serotonin receptor agonists and primarily stimulate the 5-hydroxytryptamine (HT)2A receptor (4, 6). Classic psychedelic drugs give rise to altered perception, especially visual perception, changes in affect in the direction of both ecstasy and anxiety, altered time perception, audiovisual synaesthesia, derealisation and depersonalisation, as well as pseudohallucinations (i.e. hallucinations with preserved awareness of reality) (7). The effects of the most studied psychedelic drugs, psilocybin and LSD, last for approximately 6 and 12 hours, respectively (4). Repeated use leads to tolerance owing to 5-HT2A receptor downregulation (4).
It has been argued that classic psychedelic drugs can have dangerous adverse effects, but these claims are based on studies with questionable methodology (4). Systematic studies to date show the drugs to have low toxicity and a high therapeutic index (4). Classic psychedelic drugs pose minimal risk of addiction (4). In a ranking of 20 legal and illegal substances on the basis of harmful effects for the individual user and society, alcohol was ranked bottom, whereas psilocybin and LSD were among the least harmful (5). The latter have little impact on the dopaminergic system, which may explain the low risk for development of dependence (4). However, there are also case reports describing serious but rare and mainly transient adverse effects, such as rhabdomyolysis, lower extremity ischaemia and cortical blindness following recreational use (4). The use of psychedelic drugs of uncontrolled strength and purity, and the tendency for multiple psychoactive drugs to be used simultaneously, complicates the interpretation of such case reports (4). Further, the risk of these complications in a controlled clinical environment appears to be low. These adverse effects have not been observed in modern clinical trials of classic psychedelic drugs in selected patient populations (8–16).
Hallucinogen Persisting Perception Disorder (HPPD) is a condition that has primarily been associated with recreational use of LSD (17). The condition is characterised by recurring perceptual symptoms long after the acute effects of the drug have worn off. Its incidence is unknown, but it is considered rare. Studies of the condition have methodological weaknesses and are based largely on case reports (17), and the condition has not been observed in modern studies in the field (8–16). An American study with 130 000 participants, of whom 13.4 % reported current and/or previous use of psychedelics (LSD, psilocybin or mescaline), showed no association between usage and mental health problems (18). A larger and more recent population study found that the risk of mental disorders including suicidal thoughts was reduced among users of classic psychedelic drugs, whereas it was increased among users of other illegal substances (19).
Method
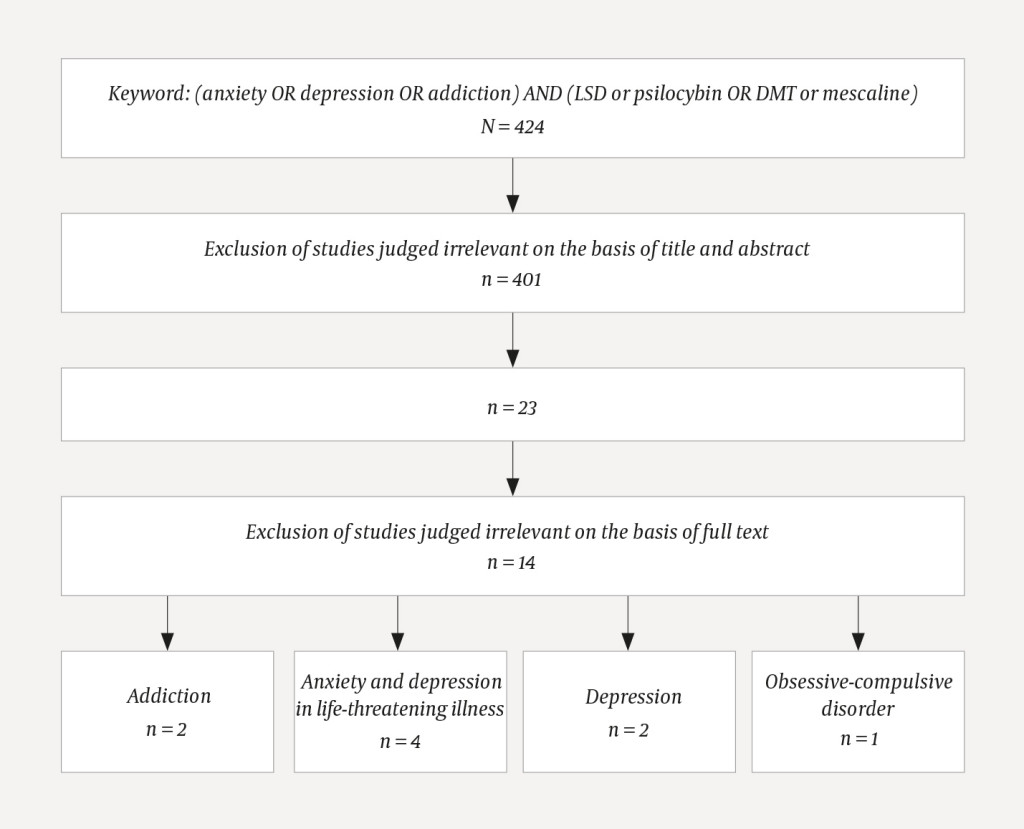
We conducted a literature review of the treatment of mental disorders with classic psychedelic drugs, identifying relevant articles through PubMed. The search was limited to the period from 1 January 1990 to 31 December 2017 to ensure a focus on modern studies using methods that comply with current standards for evidence-based medicine. We used the keywords 'anxiety' OR 'depression' OR 'addiction', in combination with 'psilocybin' OR 'LSD' OR 'DMT' OR 'mescaline'. The search algorithm is shown in Figure 1. The articles identified were manually reviewed as follows: The inclusion criteria were English-language original articles in which clinical studies had been conducted on patients. Exclusion criteria were population studies, review articles and studies on healthy volunteers. Other drugs with psychedelic properties, such as ketamine and MDMA (3,4-methylenedioxymethamphetamine) were not included. All studies reviewed in the results section of the current article were identified through the above search.
Results
Of a total of 424 articles, nine fulfilled the inclusion criteria (8–16). These could be divided into four main categories: anxiety and depression in life-threatening illness (four studies), depression (two studies), addiction (two studies) and obsessive-compulsive disorder (one study). In all studies, psychedelic drugs were administered in combination with psychological treatment. The studies are summarised in Table 1.
Table 1
Overview and comparison of the nine studies identified through the literature search. HAM-A: Hamilton Anxiety Rating Scale; HAMD: Hamilton Rating Scale For Depression; HADS: Hospital Anxiety and Depression Scale; STAI: StateTrait Anxiety Inventory; BDI: Beck's Depression Inventory; QIDS: Quick Inventory of Depressive Symptoms; YBOCS: Yale-Brown Obsessive Compulsive Scale.
| Study |
Number of patients, condition |
Design, follow-up time |
Intervention vs. control group |
Primary endpoint |
Response rate |
Remission rate |
Effect size1 |
|---|---|---|---|---|---|---|---|
| Ross 2016 (14) |
29, anxiety and depression in life-threatening cancer |
Randomised controlled double-blind crossover study, 6 months |
Psilocybin (0.3 mg/kg) |
HADS |
BDI: |
BDI: |
Different effect sizes for different endpoints: 0.80–1.69 prior to crossover2 |
| Griffiths 2016 (10) |
51, anxiety and depression in life-threatening cancer |
Randomised controlled double-blind crossover study, 6 months |
Psilocybin |
HAMD (depression) HAM-A (anxiety) |
HAMD: |
HAMD: High dose: 60 % |
0.82 prior to crossover. |
| Grob 2011 (11) |
12, anxiety in advanced cancer |
Controlled double-blind crossover pilot study, 6 months |
Psilocybin (0.2 mg/kg) |
Multiple parameters, but no clearly defined primary endpoint |
Not stated |
Not stated |
Not stated |
| Gasser 2014 (16) |
12, anxiety in life-threatening illness |
Randomised controlled double-blind pilot study, 12 months |
LSD 200 μg |
STAI (state and trait anxiety) |
Not stated |
Not stated |
State anxiety: |
| Carhart-Harris 2016 (9) |
12, depression |
Open pilot study, 3 months |
Psilocybin |
QIDS |
67 % |
58 % |
3.1 one week and 2.0 three months after treatment compared with baseline3 |
| Osório 2015 (15) |
6, depression |
Open pilot study, 3 weeks |
Ayahuasca |
Multiple parameters, but no clearly defined primary endpoint |
Not stated |
Not stated |
Not stated |
| Johnson 2014 (12) |
15, nicotine addiction |
Open pilot study, 6 months |
Psilocybin |
Multiple parameters, but no clearly defined primary endpoint |
Not stated |
Not stated |
Not stated |
| Bogenschutz 2015 (8) |
10, alcohol addiction |
Open pilot study, 36 weeks |
Psilocybin |
Percentage of days with alcohol consumption |
Not stated |
Not stated |
0.75–1.382 |
| Moreno 2006 (13) |
9, obsessive-compulsive disorder |
Pilot study, 6 months |
Psilocybin |
YBOCS |
Not stated |
Not stated |
Not stated |
1All studies showed significant differences (p < 0.05) in at least one outcome
2Cohen's d
3Hedges' g
4The drink contained 0.8 mg/ml N,N-dimethyltryptamine (DMT) and 0.21 mg/ml harmine
Anxiety and depression in cases of life-threatening illness
Anxiety and depression increase morbidity and hasten death in cancer patients. The treatment options available are often ineffective (10). In a double-blind controlled crossover pilot study in 12 patients with anxiety and advanced cancer (11), patients received either a moderate dose of psilocybin (0.2 mg/kg) or niacin, which triggers a mild physiological reaction but no psychological effects, prior to crossover. Depression measured with the Beck Depression Inventory (BDI) improved after psilocybin treatment with the effect becoming significant after six months. Anxiety as measured by the State Trait Anxiety Inventory (STAI) was also reduced, with the effect significant one and three months after treatment.
A double-blind randomised controlled pilot study in 12 patients with anxiety and a life-threatening illness, most of whom (72.7 %) had cancer, examined the efficacy of 200 μg or 20 μg of LSD. The results demonstrated a positive trend towards reduced anxiety, both state and trait, measured with the STAI scale. The reduction in anxiety was still present at a 12-month follow-up (16).
In a double-blind randomised controlled crossover study, 51 patients with life-threatening cancer and anxiety and/or depression received either high-dose or low-dose psilocybin prior to crossover (10). All patients fulfilled the criteria for a mental disorder according to the Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV), including adjustment disorder, generalised anxiety disorder, dysthymia and depressive episodes. All patients received both low- and high-dose psilocybin separated by an interval of approximately five weeks. Significant differences were seen between the groups, with reductions in depression and anxiety and improvements in quality of life and greater acceptance of death. The differences were confirmed by clinicians, the patients themselves and their relatives. The primary endpoints on the Hamilton Anxiety Rating Scale (HAM-A) and the Hamilton Scale for Depression (HAMD) were evaluated by clinicians. The effects were still present at follow-up six months after treatment: 77–83 % of patients continued to show a response and 59–71 % were in remission. Blinding provided some protection against expectation effects.
In a double-blind randomised controlled crossover study, 29 patients with cancer-related anxiety and depression received either psilocybin or niacin prior to crossover (14). All patients fulfilled the criteria for a mental disorder according to DSM-IV: 90 % had adjustment disorder and 10 % had generalised anxiety disorder. Significant differences were detected between the groups prior to the crossover, for both anxiety and depression. Six months after treatment, 60–80 % of patients had maintained a response to psilocybin according to the Beck Depression Inventory and the Hospital Anxiety and Depression Scale (HADS).
Depression
A large proportion (20–30 %) of patients with unipolar depression are treatment-resistant (2). In an open study of 12 patients with treatment-resistant depression, defined as no improvement after at least six weeks of treatment with at least two types of antidepressants, 8 out of 12 patients achieved remission as measured with the Quick Inventory of Depressive Symptoms (QIDS) one week after treatment with psilocybin (9). At a follow-up appointment after three months, 5 out of 12 patients were still in remission and 7 out of 12 showed a continued response (9).
An open study of ayahuasca in six patients with recurrent depression demonstrated an immediate antidepressant effect that was statistically significant and that persisted at follow-up one and three weeks (15) after treatment. Two weeks after treatment, a non-significant increase in symptoms was observed. Studying the potential antidepressant efficacy of ayahuasca is challenging because the drink contains both the psychedelic drug N,N-dimethyltryptamine and a monoamine oxidase inhibitor, the latter being an established group of antidepressants. It is difficult to envisage extensive use of ayahuasca in clinical trials until a standardised preparation of the active ingredients is available (4).
Addiction
In an open study of psilocybin in ten patients with alcohol dependence, alcohol consumption was significantly reduced from baseline to follow-up at 36 weeks (8).
In an open study of psilocybin in 15 patients with treatment-resistant tobacco/nicotine addiction (12), 80 % of participants were abstinent after six months. This was confirmed by objective measures of smoking cessation.
Obsessive-compulsive disorder
In an open study of psilocybin in nine patients with obsessive-compulsive disorder, most patients experienced a reduction of core symptoms. However, expectation effects appear likely given the lack of correlation between dose and response, and the efficacy of the very low minimum dose (13).
Adverse effects
None of the nine studies reviewed reported serious adverse effects. A pooled analysis of eight double-blind randomised controlled trials with a total of 110 healthy volunteers showed that psilocybin appears to be well tolerated: 7 % of participants receiving the highest doses experienced acute adverse effects, but these were managed successfully by supportive healthcare personnel and without the use of emergency medications. There were no long-term adverse effects (7).
Persistent psychotic symptoms are very rare. Only a single case has been reported among 1 200 healthy volunteers of a psychotic reaction lasting more than 48 hours, and this person had a monozygotic twin with schizophrenia (20). Psychotic disorders in participants and their first or second degree relatives are exclusion criteria for modern studies (21). Persistent psychotic symptoms have not been observed in modern day studies in the field (8–16).
Discussion
The key findings from the studies in this literature review support the idea that one or a few doses of a classic psychedelic drug, most commonly psilocybin, have immediate and sustained efficacy in multiple mental disorders. None of the nine studies reported serious adverse effects. The two largest randomised controlled trials (10, 14) independently reached the same conclusion, namely that psilocybin has significant and lasting (six months) efficacy against cancer-related anxiety and depression. Altogether the studies show that psilocybin and LSD are efficacious against anxiety and depression in cases of life-threatening illness (10, 11, 14, 16).
All of the studies discussed have methodological limitations, related to design, blinding, unclearly defined endpoints, patient selection and/or sample size. It is not possible to confirm hypotheses about the efficacy of psychedelic drugs in open studies without a control group or in the open phases of randomised controlled trials. Without a control arm, it is also difficult to distinguish the effects of drugs from those of the psychological treatment given in combination. The review thus shows that it is difficult to use the results of current studies as a basis for changes in clinical practice.
The results suggest that psychedelic drugs have potential clinical efficacy with few adverse effects in a controlled clinical setting. There are therefore grounds for conducting high-quality systematic studies, in which it will be important to:
distinguish psychological from pharmacological effects
implement a protocol without selection bias in personnel or participants
address problems related to blinding and expectation effects, for example through the use of a low dose rather than a standard placebo
check for acute and long-term somatic and psychological adverse effects (6)
Psychedelic drugs must be investigated in more and larger studies to determine whether they have clinical efficacy in the treatment of mental disorders. It will be important to keep psychological therapy to a minimum to enable drug effects to be distinguished from those of psychological therapy, without compromising the need for patients to feel safe and supported.
If the existing results can be confirmed in future studies, the immediate and sustained efficacy of a single dose will introduce a new principle in psychiatric treatment. This is similar to the results seen in the testing of ketamine in depression, although those effects lasted for a few weeks (22). There are strong indications that it would be wise to begin studies of psychedelic drugs in severe forms of depression, including treatment-resistant depression. This is a major clinical problem with few or no effective treatment options (6).
Current antidepressant drugs only begin to work after a few weeks and must be taken daily. A treatment effect with more rapid onset would be advantageous. Sustained efficacy of a single dose would also be beneficial compared to the daily administration of drugs and the associated adverse effects. Furthermore, psychedelic drugs have a different mechanism of action to currently available antidepressants, namely 5-HT2A agonism as opposed to predominantly monoamine reuptake inhibition (4).
There are now several phase II studies of psychedelic drugs in the planning stages or underway according to the ClinicalTrials.gov database. Psilocybin will be tested in depression, obsessive-compulsive disorder and in nicotine, alcohol and cocaine addiction. LSD will be tested in anxiety in the presence and absence of life-threatening illness (23). This is an exciting development. We need new evidence-based treatment options for mental disorders.
Main message
There is growing interest in clinical trials of classic psychedelic drugs for the treatment of mental disorders
Classic psychedelic drugs appear to pose little risk of serious adverse effects
Classic psychedelic drugs, especially psilocybin, have been tested in several recent clinical trials, with promising results
Classic psychedelic drugs should be tested in systematic clinical trials to determine their therapeutic potential
- 1.
Grof S, Goodman LE, Richards WA et al. LSD-assisted psychotherapy in patients with terminal cancer. Int Pharmacopsychiatry 1973; 8: 129 - 44. [PubMed][CrossRef]
- 2.
Rucker JJ, Jelen LA, Flynn S et al. Psychedelics in the treatment of unipolar mood disorders: a systematic review. J Psychopharmacol 2016; 30: 1220 - 9. [PubMed][CrossRef]
- 3.
Krebs TS, Johansen PØ. Lysergic acid diethylamide (LSD) for alcoholism: meta-analysis of randomized controlled trials. J Psychopharmacol 2012; 26: 994 - 1002. [PubMed][CrossRef]
- 4.
Nichols DE. Psychedelics. Pharmacol Rev 2016; 68: 264 - 355. [PubMed][CrossRef]
- 5.
Nutt DJ, King LA, Phillips LD. Drug harms in the UK: a multicriteria decision analysis. Lancet 2010; 376: 1558 - 65. [PubMed][CrossRef]
- 6.
Carhart-Harris RL, Goodwin GM. The therapeutic potential of psychedelic drugs: past, present, and future. Neuropsychopharmacology 2017; 42: 2105 - 13. [PubMed][CrossRef]
- 7.
Studerus E, Kometer M, Hasler F et al. Acute, subacute and long-term subjective effects of psilocybin in healthy humans: a pooled analysis of experimental studies. J Psychopharmacol 2011; 25: 1434 - 52. [PubMed][CrossRef]
- 8.
Bogenschutz MP, Forcehimes AA, Pommy JA et al. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol 2015; 29: 289 - 99. [PubMed][CrossRef]
- 9.
Carhart-Harris RL, Bolstridge M, Rucker J et al. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry 2016; 3: 619 - 27. [PubMed][CrossRef]
- 10.
Griffiths RR, Johnson MW, Carducci MA et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J Psychopharmacol 2016; 30: 1181 - 97. [PubMed][CrossRef]
- 11.
Grob CS, Danforth AL, Chopra GS et al. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry 2011; 68: 71 - 8. [PubMed][CrossRef]
- 12.
Johnson MW, Garcia-Romeu A, Cosimano MP et al. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol 2014; 28: 983 - 92. [PubMed][CrossRef]
- 13.
Moreno FA, Wiegand CB, Taitano EK et al. Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J Clin Psychiatry 2006; 67: 1735 - 40. [PubMed][CrossRef]
- 14.
Ross S, Bossis A, Guss J et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol 2016; 30: 1165 - 80. [PubMed][CrossRef]
- 15.
Osório FL, Sanches RF, Macedo LR et al. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a preliminary report. Rev Bras Psiquiatr 2015; 37: 13 - 20. [PubMed][CrossRef]
- 16.
Gasser P, Holstein D, Michel Y et al. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis 2014; 202: 513 - 20. [PubMed][CrossRef]
- 17.
Orsolini L, Papanti GD, De Berardis D et al. The "endless trip" among the NPS users: psychopathology and psychopharmacology in the hallucinogen-persisting perception disorder. a systematic review. Front Psychiatry 2017; 8: 240. [PubMed][CrossRef]
- 18.
Krebs TS, Johansen PØ. Psychedelics and mental health: a population study. PLoS One 2013; 8: e63972. [PubMed][CrossRef]
- 19.
Hendricks PS, Thorne CB, Clark CB et al. Classic psychedelic use is associated with reduced psychological distress and suicidality in the United States adult population. J Psychopharmacol 2015; 29: 280 - 8. [PubMed][CrossRef]
- 20.
Cohen S. Lysergic acid diethylamide: side effects and complications. J Nerv Ment Dis 1960; 130: 30 - 40. [PubMed][CrossRef]
- 21.
Johnson M, Richards W, Griffiths R. Human hallucinogen research: guidelines for safety. J Psychopharmacol 2008; 22: 603 - 20. [PubMed][CrossRef]
- 22.
Quintana DS, Steen NE, Andreassen OA. The promise of intranasal esketamine as a novel and effective antidepressant. JAMA Psychiatry 2018; 75: 123 - 4. [PubMed][CrossRef]
- 23.
ClinicalTrials.gov. U.S. National Library of Medicine. https://www.clinicaltrials.gov/ct2/results?cond=&term=psychedelic&cntry=&state=&city=&dist (30.8.2018).