The prevalence of multiple sclerosis is increasing and it is estimated that about 10 000 people in Norway have the disease (1). Multiple sclerosis reduces the lifespan by 5 – 10 years and is a frequent cause of neurological disability. Despite improved treatment, there is therefore still a need for information that could help to prevent the disease.
Research in epidemiology, genetics and immunology has provided greater insight into the factors that can cause multiple sclerosis. The aim of the current article is to discuss environmental impact on disease risk and disease progression.
Method
The article is based on selected English-language original and review articles, identified through a literature search in PubMed using the search term «multiple sclerosis» combined with «environment» (1 523 hits), «vitamin D» (760 hits), «EBV» (317 hits), «Epstein-Barr virus» (544 hits), «smoking» (336 hits), «nicotine» (21 hits), «cotinine» (5 hits), «vitamin A» (47 hits), «retinol» (53 hits), «sodium» (398 hits), «body mass» (188 hits) and «obesity» (139 hits). The search was terminated in September 2014.
The combined knowledge base of the authors has been built up through years of interest and work in the area. Titles and abstracts have been reviewed, and full-text versions of articles examined in the majority of cases. When assessing epidemiological evidence, we have placed particular weight on prospective cohort studies and large case-control studies. When assessing impact on disease course, we have weighted intervention studies and observational studies according to methodological quality.
We have placed particular emphasis on systematic reviews of treatment effects and have evaluated the original articles upon which these are based. Where clinical trials have been summarised in methodologically high-quality systematic reviews, the latter were chosen as references. Regarding mechanisms of action, we have prioritised studies with human data, as well as in vivo studies in animal models that we believe to be particularly relevant.
Heredity and environment
Heritability is a measure of the proportion of variation in a trait in a population that is due to genetic variation. The heritability of multiple sclerosis varies from 25 % to 76 % across studies (2). Large genome-wide association studies have revealed that variation in more than 100 genes contributes to disease risk (3). However, each known gene variant or environmental factor produces only a modest increase in risk, leading many to argue that there must be interactions between genes and environmental factors (4).
It has been shown, for example, that smoking and obesity have the greatest impact on multiple sclerosis risk among carriers of HLA genes that predispose to the disease (5, 6). HLA genes play an important role in the immune response, and this finding may thus indicate that obesity and smoking increase disease risk via effects on the immune system. One possible connection between genes and environment is epigenetics, whereby environmental factors affect gene expression without changing the nucleotide sequence of the DNA strand.
Biological siblings of persons with multiple sclerosis have an increased disease risk, while adopted siblings have the same risk as the general population (7). This indicates that environmental factors relevant to multiple sclerosis do not selectively affect those who become ill, but are widespread wherever the disease is common. Such factors can be difficult to detect because most of those who do not become ill will also have been exposed.
Distribution
The prevalence of multiple sclerosis is low around the equator and increases with increasing latitude, even after adjustment for the strongest genetic risk factor for the disease – the HLA-DRB1*1501 allele (8). Data from Europe (9) and North America (10) indicate, however, that this gradient is decreasing due to increasing incidence in the south. Epidemiological changes such as these may be indicative of altered exposure to environmental factors, as may the observed increase in the proportion of women who are developing multiple sclerosis (11).
Studies from various countries have shown that those born in the spring have a slightly higher risk of multiple sclerosis than those born in the autumn (12). This may suggest that environmental factors act very early on in life and are related to the seasons, as in infectious diseases and sunlight. These studies have been criticised for not having adjusted for year and place of birth (13). Nevertheless, we still found a significant excess of April birthdays among patients with multiple sclerosis after adjusting for these factors (14).
Studies have shown that migration from areas of high to low prevalence is associated with reduced disease risk (15), particularly if the move occurs during childhood or early adolescence. Migrants who returned to the West Indies after having lived in France had an increased disease risk, especially if they returned before they were 15 years old (16). Similarly, a study in Oslo showed that multiple sclerosis was more frequent among immigrants from the Middle East and Asia than disease prevalence in these countries would predict (17). Although such studies are hampered by uncertainties related to differences in diagnostics and the fact that emigrants are a selected group, together these migration studies reveal the importance of environmental factors in early life.
Selected environmental factors
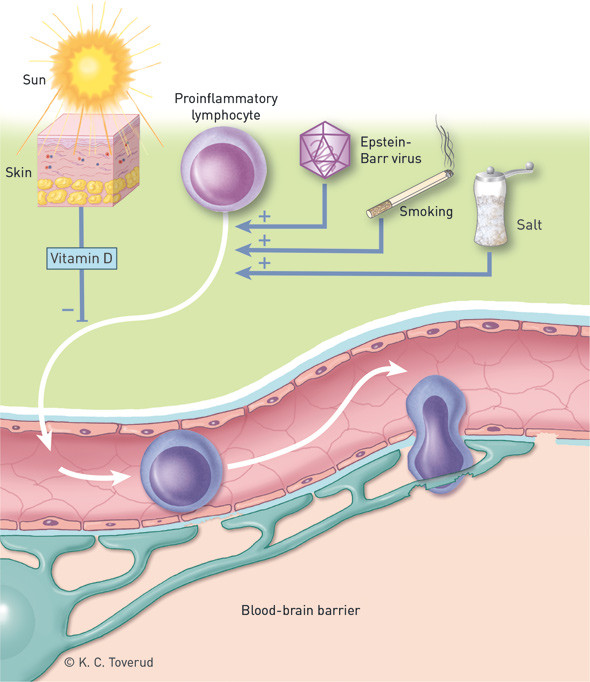
Epstein-Barr virus
Despite the fact that previous Epstein-Barr virus infection is very common in the general adult population (90 – 95 %), this virus is strongly associated with the development of multiple sclerosis (18). Virtually all adults with the disease have been infected, and a prospective study showed that infection occurs before the onset of multiple sclerosis symptoms (19). Previous infection also increases the risk of multiple sclerosis in children, but not all children with the disease are seropositive for Epstein-Barr virus (20). The infection is therefore probably not necessary for developing multiple sclerosis.
Two factors suggest that adolescence is the most important critical period for Epstein-Barr virus exposure: in areas where primary infection occurs early, the prevalence of multiple sclerosis is low, and infectious mononucleosis, which is caused by primary Epstein-Barr virus infection during adolescence, doubles the risk of multiple sclerosis (18).
Possible mechanisms by which Epstein-Barr virus infection may predispose to multiple sclerosis include immunological cross-reaction, that the virus protects autoreactive B cells from apoptosis, and activation of autoreactive T cells by an excessive immune response against the Epstein-Barr virus (18). In accordance with an immunological mechanism, it seems that T cells directed against the virus accumulate in the cerebrospinal fluid of persons with multiple sclerosis (21). Two studies have detected Epstein-Barr virus-infected B cells in brain tissue from deceased individuals who had multiple sclerosis (22, 23). However, this has been difficult to reproduce (24), and does not seem to be specific to multiple sclerosis (23).
Smoking
A number of epidemiological studies suggest that smoking doubles the risk of multiple sclerosis (25), and that there is a dose-response relationship with cumulative smoking dose (26). The increase in disease risk is also supported by the fact that elevated levels of cotinine (a surrogate marker of nicotine) were detected in blood samples taken before the development of multiple sclerosis (27). Nicotine per se, however, is probably not a key pathogenic factor, given that use of snus does not seem to increase disease risk (28) and that nicotine protects against the development of multiple sclerosis-like disease in mice (29). The critical period in the case of smoking is unclear. One study found no effect of age (26), while another observed increased susceptibility to multiple sclerosis in individuals exposed to smoking during adolescence/young adulthood (27).
The effect of smoking on disease activity is also unclear. Smoking is associated with faster transition from symptom onset to clinically definite multiple sclerosis (30), more rapid disability progression (31) and increased disease activity as measured by MRI (32). However, a meta-analysis showed only a borderline significant association with disease progression (25).
Possible mechanisms of action for smoking include toxic effects on neurons and oligodendrocytes and activation of encephalitogenic lymphocytes in the lungs (33).
Vitamin D
The hypothesised link between vitamin D and multiple sclerosis stems from the observed inverse relationship between disease prevalence and sunlight intensity (34). The vitamin D hypothesis is supported by a number of studies in vitro and in animal models, which have shown that active vitamin D (1,25-dihydroxyvitamin D/calcitriol) induces anti-inflammatory immune cells, including regulatory T cells (35).
A prospective study in American nurses found that those who took dietary supplements with over 400 international units (IU) of vitamin D per day had a 40 % lower risk of multiple sclerosis than those who did not use supplements (36). In another large prospective American study, the fifth of participants with the highest vitamin D levels (> 99 nmol/l) had a 60 % lower risk of multiple sclerosis than those with the lowest levels (< 63 nmol/l) (37). Similar results have been obtained in Sweden (38).
Prospective studies also suggest that higher vitamin D levels are associated with a lower risk of relapse (39) and low disease activity as shown by MRI (40), as well as a lower risk of developing multiple sclerosis after the first clinical signs of demyelinating disease (41).
Other factors
Sunlight may have immunomodulatory properties independent of vitamin D (42). Epidemiological studies suggest that limited sun exposure may be an independent risk factor for multiple sclerosis (43) – (45), and it is therefore difficult to distinguish the effects of vitamin D from other effects of sunlight.
As with other immune-mediated disorders, it has been suggested that early exposure to large numbers of microbes protects against multiple sclerosis in later life. This so-called hygiene hypothesis is consistent with the lower incidence of the disease in developing countries compared to countries with better socioeconomic conditions (46), but is difficult to verify in studies.
Obesity prior to adulthood is associated with increased risk of multiple sclerosis (47, 48). Obesity results both in lower circulating levels of vitamin D due to increased distribution of the vitamin in adipose tissue, and higher serum levels of proinflammatory cytokines (particularly interleukin-6 and tumour necrosis factor-alpha), both of which could conceivably contribute to the increased risk.
Vitamin A has immunoregulatory properties that may be relevant to multiple sclerosis. A prospective study showed that intermediate levels of retinol binding protein (a surrogate marker of vitamin A) were associated with low disease risk (49). Moreover, we found that increased levels of retinol were associated with a lower risk of disease activity as revealed by MRI (50).
Mice with large amounts of salt in their diets were recently shown to develop a more aggressive multiple sclerosis-like disease than mice that consumed little salt (51), while patients with multiple sclerosis with high salt intake had significantly higher disease activity than patients with low intake (52). High salt intake in mice stimulated Th17 cells, which have been implicated in several autoimmune diseases (51).
Discussion
Association is not synonymous with causality. Table 1 summarises the various points that argue in favour of causality for each of the proposed environmental factors, based on evaluation with a set of commonly used criteria (Box 1) (53).
Table 1
Putative environmental factors in multiple sclerosis. Evidence for causality as assessed using the factors proposed by Hill (53) (see Box 1). Based on the authors’ evaluation: +: Existing studies consistent with Hill’s viewpoint. –: Insufficient evidence from existing studies or no studies available
| Hill |
Epstein-Barr virus |
Smoking |
Vitamin D |
Obesity |
Vitamin A |
Salt intake |
| Strength |
+ |
– |
– |
– |
– |
– |
| Consistency |
+ |
+ |
+ |
+ |
– |
– |
| Temporal relationship¹ |
+ |
+ |
+ |
+ |
– |
– |
| Biological gradient |
– |
+ |
+ |
– |
– |
– |
| Biological rationale |
+ |
+ |
+ |
+ |
+ |
+ |
| Coherence |
+ |
+ |
+ |
+ |
+ |
+ |
| Randomised studies |
– |
– |
– |
– |
– |
– |
| [i] | ||||||
[i] ¹ Temporal relationships are evaluated on the basis of prospective studies. However, this is difficult in multiple sclerosis because it is unclear when the disease process starts.
In 1965, the epidemiologist Sir Austin Bradford Hill proposed that the following factors should be emphasised when assessing causality (53)
Strength of the association – a weak association does not preclude a causal relationship, but the stronger the association, the greater the likelihood of causality
Consistency – concurrent findings in different populations, with the use of different study designs, in different situations
Specificity – causality is more likely if a particular exposure gives one specific outcome, which in medicine is very rare¹
Temporal relationship – exposure must have occurred before the start of the disease process
Biological gradient – increased exposure gives an increased incidence or risk of disease, or faster disease progression, and vice versa for inverse associations
Biological rationale – a biologically plausible relationship increases the likelihood of causality. However, this will always be constrained by the established knowledge of the time
Coherence – any causal relationship should not be contrary to established knowledge. Concurrent findings in epidemiological studies and laboratory experiments strengthen the case for causality
Experiment – does the disease develop after exposure to the factor or can it be prevented by eliminating exposure? In the current context, the appropriate experiment would be a (randomised) clinical trial
Analogy – exposure to similar factors has similar effects¹
¹ Many, including Sir Austin Bradford Hill himself, place less importance on «specificity» and «analogy».
The criterion upon which most weight is placed – effect on disease risk in a randomised clinical trial – has not been examined for any environmental factor in multiple sclerosis. Primary prophylaxis is complicated because multiple sclerosis is a rare and multifactorial disease, and because long periods of time can probably elapse between exposure and symptom onset. While interventions targeted at smoking would be exclusively beneficial for health, measures directed against Epstein-Barr virus and vitamin D could also have unintended negative health effects, and studies may well require very large groups and long follow-up periods with measurement of both positive and negative outcomes. Nevertheless, on the basis of consistent findings in epidemiological and experimental studies, some epidemiologists believe that Epstein-Barr virus, vitamin D and smoking are causal factors (54).
There is considerable interest in the use of vitamin D for secondary prophylaxis in multiple sclerosis. Several underpowered pilot studies have been performed, but a meta-analysis recently concluded that high-dose vitamin D supplements have no effect on disease activity (55).
Based on current knowledge, we would not recommend large doses of vitamin D in cases of multiple sclerosis. However, patients often have vitamin D levels that are suboptimal for bone health – they develop early osteopenia and have an increased risk of bone fractures (56, 57). Repeated measurements of vitamin D level in 88 Norwegians with multiple sclerosis revealed that most had serum levels below official recommendations (50 nmol/l) throughout much of the year (58).
From what is known about the effect of supplements on serum levels, we have calculated that a moderate supplement of 800 IU (20 μg) of vitamin D₃ daily would have given almost all an acceptable level (> 50 nmol/l) throughout the year, and a level associated with low disease activity (75 – 125 nmol/l) for most of the year (58). We would therefore recommend supplements of about 800 IU for people with multiple sclerosis. Alternatively, the concentration of 25-hydroxyvitamin D in serum can be measured, and levels between 75 nmol/l and 125 nmol/l sought.
MAIN MESSAGE
The prevalence of multiple sclerosis is increasing, both in Norway and in most Western countries.
The increase could reflect altered exposure to environmental factors.
Epstein-Barr virus, smoking and vitamin D deficiency are the environmental factors that are most strongly associated with the risk of developing the disease.
- 19.
Levin LI, Munger KL, O’Reilly EJ et al. Primary infection with the Epstein-Barr virus and risk of multiple sclerosis. Ann Neurol 2010; 67: 824 – 30. [PubMed]
- 29.
Naddafi F, Reza Haidari M, Azizi G et al. Novel therapeutic approach by nicotine in experimental model of multiple sclerosis. Innov Clin Neurosci 2013; 10: 20 – 5. [PubMed]
- 34.
Goldberg P. Multiple sclerosis: vitamin D and calcium as environmental determinants of prevalence (a viewpoint) Part 1: sunlight, dietary factors and epidemiology. Int J Environ Stud 1974; 6: 19 – 27. [CrossRef]
- 39.
Simpson S jr., Taylor B, Blizzard L et al. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol 2010; 68: 193 – 203. [PubMed]
- 53.
Hill AB. The Environment and disease: association or causation? Proc R Soc Med 1965; 58: 295 – 300. [PubMed]